High-efficiency secretory expression and characterization of the recombinant type III human-like collagen in Pichia pastoris
- PMID: 38647563
- PMCID: PMC10992891
- DOI: 10.1186/s40643-022-00605-4
High-efficiency secretory expression and characterization of the recombinant type III human-like collagen in Pichia pastoris
Abstract
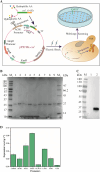
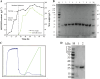
Collagen, the highest content protein in the body, has irreplaceable biological functions, and it is widespread concerned in food, beauty, and medicine with great market demand. The gene encoding the recombinant type III human-like collagen α1 chain fragment was integrated into P. pastoris genome after partial amino acids were substituted. Combined with promoter engineering and high-density fermentation technology, soluble secretory expression with the highest yield of 1.05 g L-1 was achieved using two-stage feeding method, and the purity could reach 96% after affinity purification. The determination of N/C-terminal protein sequence were consistent with the theoretical expectation and showed the characteristics of Gly-X-Y repeated short peptide sequence. In amino acid analysis, glycine shared 27.02% and proline 23.92%, which were in accordance with the characteristics of collagen. Ultraviolet spectrum combined with Fourier transform infrared spectroscopy as well as mass spectrometry demonstrated that the target product conformed to the characteristics of collagen spectrums and existed as homologous dimer and trimer in the broth. This work provided a sustainable and economically viable source of the recombinant type III human-like collagen.
Keywords: Characterization; Fermentation; Human-like collagen; Protein purification; Secretory expression.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Expression, characterization, and application of human-like recombinant gelatin.Bioresour Bioprocess. 2024 Jul 17;11(1):69. doi: 10.1186/s40643-024-00785-1. Bioresour Bioprocess. 2024. PMID: 39014092 Free PMC article.
-
New strategy for expression of recombinant hydroxylated human collagen α1(III) chains in Pichia pastoris GS115.Biotechnol Appl Biochem. 2015 May-Jun;62(3):293-9. doi: 10.1002/bab.1264. Epub 2015 Mar 23. Biotechnol Appl Biochem. 2015. PMID: 24953863
-
[Preparation and characterization of recombinant human-source collagen].Sheng Wu Gong Cheng Xue Bao. 2019 Feb 25;35(2):319-326. doi: 10.13345/j.cjb.180266. Sheng Wu Gong Cheng Xue Bao. 2019. PMID: 30806062 Chinese.
-
Secretory expression of human protein in the Yeast Pichia pastoris by controlled fermentor culture.Recent Pat Biotechnol. 2010 Jun;4(2):153-66. doi: 10.2174/187220810791110679. Recent Pat Biotechnol. 2010. PMID: 20180764 Review.
-
New Developments in Pichia pastoris Expression System, Review and Update.Curr Pharm Biotechnol. 2018;19(6):451-467. doi: 10.2174/1389201019666180718093037. Curr Pharm Biotechnol. 2018. PMID: 30019641 Review.
Cited by
-
Enhanced Expression of Alcohol Dehydrogenase I in Pichia pastoris Reduces the Content of Acetaldehyde in Wines.Microorganisms. 2023 Dec 25;12(1):38. doi: 10.3390/microorganisms12010038. Microorganisms. 2023. PMID: 38257867 Free PMC article.
-
Expression, characterization, and application of human-like recombinant gelatin.Bioresour Bioprocess. 2024 Jul 17;11(1):69. doi: 10.1186/s40643-024-00785-1. Bioresour Bioprocess. 2024. PMID: 39014092 Free PMC article.
-
Diffusion model assisted designing self-assembling collagen mimetic peptides as biocompatible materials.Brief Bioinform. 2024 Nov 22;26(1):bbae622. doi: 10.1093/bib/bbae622. Brief Bioinform. 2024. PMID: 39688478 Free PMC article.
-
Therapeutic proteins: developments, progress, challenges, and future perspectives.3 Biotech. 2024 Apr;14(4):112. doi: 10.1007/s13205-024-03958-z. Epub 2024 Mar 18. 3 Biotech. 2024. PMID: 38510462 Free PMC article. Review.
-
Innovations and challenges in collagen and gelatin production through precision fermentation.World J Microbiol Biotechnol. 2025 Feb 6;41(2):63. doi: 10.1007/s11274-025-04276-z. World J Microbiol Biotechnol. 2025. PMID: 39910024 Review.
References
-
- Ashraf SS, Parivar K, Hayati Roodbari N, Mashayekhan S, Amini N. Fabrication and characterization of biaxially electrospun collagen/alginate nanofibers, improved with Rhodotorula mucilaginosa sp. GUMS16 produced exopolysaccharides for wound healing applications. Int J Biol Macromol. 2022;196:194–203. doi: 10.1016/j.ijbiomac.2021.11.132. - DOI - PubMed
-
- Butkowski RJ, Noelken ME, Hudson BG. Estimation of the size of collagenous proteins by electrophoresis and gel chromatography. In: Cunningham LW, Frederiksen DW, editors. Structural and contractile proteins part A: extracellular matrix. New York: Academic Press; 1982. pp. 410–423.
-
- Duan X, Gao J, Zhou YJ. Advances in engineering methylotrophic yeast for biosynthesis of valuable chemicals from methanol. Chin Chem Lett. 2018;29:681–686. doi: 10.1016/j.cclet.2017.11.015. - DOI
Grants and funding
- No. 21978116/National Natural Science Foundation of China
- No. 32171261/National Natural Science Foundation of China
- No. 2021YFC2100900/National Key Research and Development Program of China
- No. JUSRP22047/Fundamental Research Funds for the Central Universities
- No. 2022124Z/Undergraduate Training Program for Innovation and Entrepreneurship of the Jiangnan University
LinkOut - more resources
Full Text Sources
Other Literature Sources

