Regiospecific C-H amination of (-)-limonene into (-)-perillamine by multi-enzymatic cascade reactions
- PMID: 38647597
- PMCID: PMC10992285
- DOI: 10.1186/s40643-022-00571-x
Regiospecific C-H amination of (-)-limonene into (-)-perillamine by multi-enzymatic cascade reactions
Abstract
Background: (-)-Limonene, one of cyclic monoterpenes, is an important renewable compound used widely as a key building block for the synthesis of new biologically active molecules and fine chemicals. (-)-Perillamine, as derived from (-)-limonene, is a highly useful synthon for constructing more complicated and functionally relevant chemicals.
Aim: We aimed to report a more sustainable and more efficient method for the regiospecific C-H amination of (-)-limonene into (-)-perillamine.
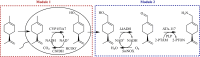
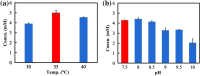
Results: Here, we report an artificial penta-enzymatic cascade system for the transformation of the cheap and easily available (-)-limonene into (-)-perillamine for the first time. This system is composed of cytochrome P450 monooxygenase, alcohol dehydrogenase and w-transaminase for the main reactions, as well as formate dehydrogenase and NADH oxidase for cofactor recycling. After optimization of the multi-enzymatic cascade system, 10 mM (-)-limonene was smoothly converted into 5.4 mM (-)-perillamine in a one-pot two-step biotransformation, indicating the feasibility of multi-enzymatic C7-regiospecific amination of the inert C-H bond of (-)-limonene. This method represents a concise and efficient route for the biocatalytic synthesis of derivatives from similar natural products.
Keywords: (−)-Limonene; (−)-Perillamine; CYP153A7; Multi-enzyme cascade; Regiospecific C–H amination; Transaminase.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
One-Pot Biocatalytic Conversion of Chemically Inert Hydrocarbons into Chiral Amino Acids through Internal Cofactor and H2O2 Recycling.Angew Chem Int Ed Engl. 2024 Nov 25;63(48):e202410260. doi: 10.1002/anie.202410260. Epub 2024 Oct 22. Angew Chem Int Ed Engl. 2024. PMID: 39187620
-
Highly selective synthesis of d-amino acids from readily available l-amino acids by a one-pot biocatalytic stereoinversion cascade.RSC Adv. 2019 Sep 23;9(51):29927-29935. doi: 10.1039/c9ra06301c. eCollection 2019 Sep 18. RSC Adv. 2019. PMID: 35531513 Free PMC article.
-
Limonene dehydrogenase hydroxylates the allylic methyl group of cyclic monoterpenes in the anaerobic terpene degradation by Castellaniella defragrans.J Biol Chem. 2018 Jun 15;293(24):9520-9529. doi: 10.1074/jbc.RA117.001557. Epub 2018 May 1. J Biol Chem. 2018. PMID: 29716998 Free PMC article.
-
Enzymatic Oxy- and Amino-Functionalization in Biocatalytic Cascade Synthesis: Recent Advances and Future Perspectives.Angew Chem Int Ed Engl. 2023 Nov 27;62(48):e202309012. doi: 10.1002/anie.202309012. Epub 2023 Sep 12. Angew Chem Int Ed Engl. 2023. PMID: 37639631 Review.
-
High-Yield Synthesis of Enantiopure 1,2-Amino Alcohols from l-Phenylalanine via Linear and Divergent Enzymatic Cascades.Org Process Res Dev. 2022 Jul 15;26(7):2085-2095. doi: 10.1021/acs.oprd.1c00490. Epub 2022 Mar 28. Org Process Res Dev. 2022. PMID: 35873603 Free PMC article. Review.
Cited by
-
Construction and optimization of a biocatalytic route for the synthesis of neomenthylamine from menthone.Bioresour Bioprocess. 2023 Nov 3;10(1):75. doi: 10.1186/s40643-023-00693-w. Bioresour Bioprocess. 2023. PMID: 38647910 Free PMC article.
References
-
- Bruggink A, Schoevaart R, Kieboom T. Concepts of nature in organic synthesis: cascade catalysis and multistep conversions in concert. Org Process Res Dev. 2003;7(5):622–640. doi: 10.1021/op0340311. - DOI
-
- Chang D, Feiten HJ, Witholt B, Li Z. Regio- and stereoselective hydroxylation of N-substituted piperidin-2-ones with Sphingomonas sp. HXN-200. Tetrahedron Asymmetry. 2002;13(19):2141–2147. doi: 10.1016/S0957-4166(02)00534-7. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

