Coupling metal and whole-cell catalysis to synthesize chiral alcohols
- PMID: 38647607
- PMCID: PMC10992956
- DOI: 10.1186/s40643-022-00560-0
Coupling metal and whole-cell catalysis to synthesize chiral alcohols
Abstract
Background: The combination of metal-catalyzed reactions and enzyme catalysis has been an essential tool for synthesizing chiral pharmaceutical intermediates in the field of drug synthesis. Metal catalysis commonly enables the highly efficient synthesis of molecular scaffolds under harsh organic conditions, whereas enzymes usually catalyze reactions in mild aqueous medium to obtain high selectivity. Since the incompatibility between metal and enzyme catalysis, there are limitations on the compatibility of reaction conditions that must be overcome.
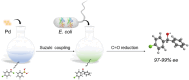
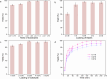
Findings: We report a chemoenzymatic cascade reaction involved Palladium (Pd) catalyzed Suzuki-Miyaura coupling and whole-cell catalyzed C = O asymmetric reduction for enantioselective synthesis of value-added chiral alcohol. The cell membrane serves as a natural barrier can protect intracellular enzymes from organic solvents.
Conclusions: With dual advantages of cascade catalysis and biocompatibility, our work provides a rational strategy to harvest chiral alcohols in high yield and excellent enantioselectivity, as a channel to establish chemoenzymatic catalysis.
Keywords: (S)-4-chlorobenzhydrol; Chemoenzymatic cascade catalysis; Chiral alcohol; Metal catalysis; Whole-cell catalysis.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Cao Y, Ge J. Hybrid enzyme catalysts synthesized by a de novo approach for expanding biocatalysis. Chin J Catal. 2021;42(10):1625–1633. doi: 10.1016/S1872-2067(21)63798-1. - DOI
-
- Huang S-H, Li W, Chen L, Xu J, Hong R. Chemoenzymatic construction of chiral alkenyl acetylenic alcohol, a key building block to access diastereoisomers of polyacetylenes. Bioresour Bioprocess. 2015;2(1):10. doi: 10.1186/s40643-014-0035-3. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

