Engineering a norcoclaurine synthase for one-step synthesis of (S)-1-aryl-tetrahydroisoquinolines
- PMID: 38647611
- PMCID: PMC10992437
- DOI: 10.1186/s40643-023-00637-4
Engineering a norcoclaurine synthase for one-step synthesis of (S)-1-aryl-tetrahydroisoquinolines
Abstract
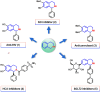
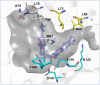
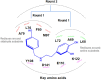
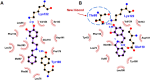
Tetrahydroisoquinoline alkaloids (THIQAs) are ubiquitous compounds with important pharmaceutical and biological activity. Their key N-heterocyclic structural motifs are synthesised via Pictet-Spengler (P-S) reaction by norcoclaurine synthases (NCS) in plants. The synthesis of 1-aryl-tetrahydroisoquinoline alkaloids has attracted increasing attention due to their antitumor and antivirus activities. Herein, the L68T/M97V mutant of NCS from Thalictrum flavum with improved activity was developed by semi-rational design. This mutant not only showed higher catalytic performance (> 96% conversion) toward benzaldehyde and dopamine over the wild-type enzyme, but also catalysed the P-S reaction of the bulky substrate 4-biphenylaldehyde and dopamine with high conversion (> 99%) for the effective synthesis of 1-aryl-THIQA. In terms of stereoselectivity, all products synthesised by the L68T/M97V mutant showed high optical purity (92-99% enantiomeric excess).
Keywords: Biocatalysis; Norcoclaurine synthase; Pictet–Spengler reaction; Protein engineering; Tetrahydroisoquinoline alkaloids.
© 2023. The Author(s).
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures





Similar articles
-
Single step syntheses of (1S)-aryl-tetrahydroisoquinolines by norcoclaurine synthases.Commun Chem. 2020 Nov 13;3(1):170. doi: 10.1038/s42004-020-00416-8. Commun Chem. 2020. PMID: 36703392 Free PMC article.
-
'Dopamine-first' mechanism enables the rational engineering of the norcoclaurine synthase aldehyde activity profile.FEBS J. 2015 Mar;282(6):1137-51. doi: 10.1111/febs.13208. Epub 2015 Feb 9. FEBS J. 2015. PMID: 25620686 Free PMC article.
-
Structural basis of enzymatic (S)-norcoclaurine biosynthesis.J Biol Chem. 2009 Jan 9;284(2):897-904. doi: 10.1074/jbc.M803738200. Epub 2008 Nov 12. J Biol Chem. 2009. PMID: 19004827
-
Norcoclaurine synthase: mechanism of an enantioselective pictet-spengler catalyzing enzyme.Molecules. 2010 Mar 24;15(4):2070-8. doi: 10.3390/molecules15042070. Molecules. 2010. PMID: 20428026 Free PMC article. Review.
-
Occurrence of Enantioselectivity in Nature: The Case of (S)-Norcoclaurine.Chirality. 2016 Mar;28(3):169-80. doi: 10.1002/chir.22566. Epub 2016 Jan 5. Chirality. 2016. PMID: 26729048 Review.
Cited by
-
Computational mechanistic investigation of the kinetic resolution of α-methyl-phenylacetaldehyde by norcoclaurine synthase.Commun Chem. 2024 Mar 27;7(1):64. doi: 10.1038/s42004-024-01146-x. Commun Chem. 2024. PMID: 38538750 Free PMC article.
References
-
- Bonamore A, Rovardi I, Gasparrini F, Baiocco P, Barba M, Molinaro C, Botta B, Boffi A, Macone A. An enzymatic, stereoselective synthesis of (S)-norcoclaurine. Green Chem. 2010;12(9):1623–1627. doi: 10.1039/c0gc00036a. - DOI
-
- Bruno E, Buemi MR, Di Fiore A, De Luca L, Ferro S, Angeli A, Cirilli R, Sadutto D, Alterio V, Monti SM, Supuran CT, De Simone G, Gitto R. Probing molecular interactions between human carbonic anhydrases (hCAs) and a novel class of benzenesulfonamides. J Med Chem. 2017;60(10):4316–4326. doi: 10.1021/acs.jmedchem.7b00264. - DOI - PubMed
Grants and funding
- 2019YFA09005000/National Key Research and Development Program of China
- 2021YFC2102800/National Key Research and Development Program of China
- 2021YFA0911400/National Key Research and Development Program of China
- 21878085/National Natural Science Foundation of China
- 31971380/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources

