Selective biosynthesis of retinol in S. cerevisiae
- PMID: 38647788
- PMCID: PMC10991881
- DOI: 10.1186/s40643-022-00512-8
Selective biosynthesis of retinol in S. cerevisiae
Abstract
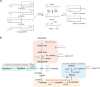
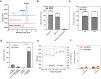
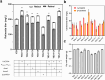
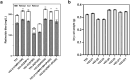
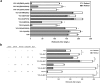
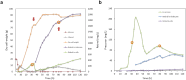
The vitamin A component retinol has become an increasingly sought-after cosmetic ingredient. In previous efforts for microbial biosynthesis of vitamin A, a mixture of retinoids was produced. In order to efficiently produce retinol at high purity, the precursor and NADPH supply was first enhanced to improve retinoids accumulation in the S. cerevisiae strain constructed from a β-carotene producer by introducing β-carotene 15,15'-dioxygenase, following by screening of heterologous and endogenous oxidoreductases for retinal reduction. Env9 was found as an endogenous retinal reductase and its activity was verified in vitro. By co-expressing Env9 with the E. coli ybbO, as much as 443.43 mg/L of retinol was produced at 98.76% purity in bi-phasic shake-flask culture when the antioxidant butylated hydroxytoluene was added to prevent retinoids degradation. The retinol titer reached 2479.34 mg/L in fed-batch fermentation. The success in selective biosynthesis of retinol would lay a solid foundation for its biotechnological production.
Keywords: Biosynthesis; Metabolic engineering; Retinal reductase; Retinol; Vitamin A.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Baker ME. Evolution of mammalian 11β- and 17β-hydroxysteroid dehydrogenases-type 2 and retinol dehydrogenases from ancestors in Caenorhabditis elegans and evidence for horizontal transfer of a eukaryote dehydrogenase to E. coli. J Steroid Biochem Mol Biol. 1998;66:355–363. doi: 10.1016/S0960-0760(98)00064-8. - DOI - PubMed
-
- Brachmann CB, Davies A, Cost GJ, et al. Designer deletion strains derived from Saccharomyces cerevisiae S288C a useful: set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast. 2016;14:115–132. doi: 10.1002/(SICI)1097-0061(19980130)14:2<115::AID-YEA204>3.0.CO;2-2. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

