Maize Aspergillus section Flavi isolate diversity may be distinct from that of soil and subsequently the source of aflatoxin contamination
- PMID: 38647834
- PMCID: PMC11258066
- DOI: 10.1007/s12550-024-00532-7
Maize Aspergillus section Flavi isolate diversity may be distinct from that of soil and subsequently the source of aflatoxin contamination
Abstract
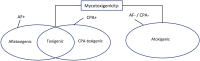
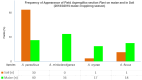
Aspergillus section Flavi (Flavi) is a diverse group of fungal species whose common members include A. flavus and A. parasiticus. These are well-known for the production of aflatoxin (AF) B and G and other toxic metabolites, like cyclopiazonic acid (CPA). They are saprophytic soil dwellers and also become crop opportunistic epiphytes. The consequence is contamination of the crop with mycotoxins, such as carcinogenic AF. We investigated the Flavi community structure of maize and that of their surrounding soil, including their mycotoxigenicity. Furthermore, we investigated the link of the maize Flavi diversity with preharvest maize AF levels. The study was carried out in four selected districts of Zambia, in a low rainfall zone. The Flavi characterisation was triphasic, involving morphological (colony colour and sclerotia formation), metabolic (AF and CPA production) and genetic (calmodulin gene polymorphism) analyses. Flavi abundance was determined by dilution plate technique on modified rose Bengal agar. Results showed that Flavi communities on maize and in soil differed. Maize had a higher Flavi species diversity than soil. A. parasiticus dominated the soil community by frequency of field appearance (85%), while maize was dominated by A. minisclerotigenes (45%). CPA-producers with or without AF production dominated the maize (65%) while producers of only AF (B/G) dominated the soil (88%). The ratio between maize A. parasiticus and A. minisclerotigenes abundance seemed to have had a bearing on the levels of AF in maize, with a ratio close to 1:1 having higher levels than a pure community of either A. parasiticus or A. minisclerotigenes.
Keywords: Aspergillus; Aflatoxin; Cyclopiazonic acid; Maize; Soil.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Abdollahi A, Buchanan R. Regulation of aflatoxin biosynthesis: induction of aflatoxin production by various carbohydrates. J Food Sci. 1981;46(2):633–635. doi: 10.1111/j.1365-2621.1981.tb04928.x. - DOI
-
- Amaranta C-C, Lethicia MA, Montet D, Lorber S, Puel O, Brabet C. Mycotoxins in Food and Beverages Innovations and Advances Part I. CRC Press; 2021. Biodiversity of aflatoxigenic Aspergillus section Flavi species according to food matrices and geographic areas; pp. 69–115.
-
- Bandyopadhyay R, Ortega-Beltran A, Akande A, Mutegi C, Atehnkeng J, Kaptoge L, Senghor AL, Adhikari BN, Cotty PJ. Biological control of aflatoxins in Africa: current status and potential challenges in the face of climate change. World Mycotoxin J. 2016;9(5):771–789. doi: 10.3920/wmj2016.2130. - DOI
-
- Benard OO, Hunja M, Isabel NW. Isolation and characterisation of aflatoxigenic Aspergillus species from maize and soil samples from selected counties of Kenya. Afr J Microbiol Res. 2013;7(34):4379–4388.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

