No requirement of perioperative glucocorticoid replacement in patients with endogenous Cushing's syndrome - a pilot study
- PMID: 38647982
- PMCID: PMC11291516
- DOI: 10.1007/s12020-024-03832-1
No requirement of perioperative glucocorticoid replacement in patients with endogenous Cushing's syndrome - a pilot study
Abstract
Purpose: Surgical therapy represents the first-line treatment for endogenous Cushing's syndrome (CS). While postoperative glucocorticoid replacement is mandatory after surgical remission, the role of perioperative glucocorticoid therapy is unclear.
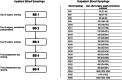
Methods: We recruited patients with central or adrenal CS in whom curative surgery was planned and patients who underwent pituitary surgery for other reasons than CS as a control group. Patients did not receive any perioperative glucocorticoids until the morning of the first postoperative day. We performed blood samplings in the morning of surgery, immediately after surgery, in the evening of the day of surgery, and in the morning of the first and third postoperative day before any morning glucocorticoid intake. We continued clinical and biochemical monitoring during the following outpatient care.
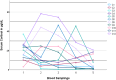
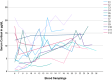
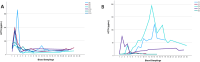
Results: We recruited 12 patients with CS (seven with central CS, five with adrenal CS) and six patients without CS. In patients with CS, serum cortisol concentrations <5.0 µg/dL (<138 nmol/L) were detected in the morning of the first and third postoperative day in four (33%) and six (50%) patients, respectively. Morning serum cortisol concentrations on the third postoperative day were significantly lower when compared to preoperative measurements (8.5 ± 7.6 µg/dL vs. 19.9 ± 8.9 µg/dL [235 ± 210 nmol/L vs. 549 ± 246 nmol/L], p = 0.023). No patient developed clinical or biochemical signs associated with hypocortisolism. During follow-up, we first observed serum cortisol concentrations >5.0 µg/dL (>138 nmol/L) after 129 ± 97 days and glucocorticoids were discontinued after 402 ± 243 days. Patients without CS did not require glucocorticoid replacement at any time.
Conclusion: Perioperative glucocorticoid replacement may be unnecessary in patients with central or adrenal CS undergoing curative surgery as first-line treatment.
Keywords: Adrenal; Cushing’s disease; Cushing’s syndrome; Hypercortisolism; Perioperative glucocorticoid replacement; Pituitary.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Rational use of glucocorticoid during pituitary surgery--a pilot study.Indian J Med Res. 2008 Sep;128(3):294-9. Indian J Med Res. 2008. PMID: 19052341 Clinical Trial.
-
Postoperative plasma cortisol levels predict long-term outcome in patients with Cushing's disease and determine which patients should be treated with pituitary irradiation after surgery.Endocr J. 2001 Feb;48(1):53-62. doi: 10.1507/endocrj.48.53. Endocr J. 2001. PMID: 11403103
-
Alterations in hypothalamic-pituitary-adrenal function immediately after resection of adrenal adenomas in patients with Cushing's syndrome and others with incidentalomas and subclinical hypercortisolism.Endocrine. 2019 Jan;63(1):140-148. doi: 10.1007/s12020-018-1769-z. Epub 2018 Sep 27. Endocrine. 2019. PMID: 30259310
-
Subclinical Cushing's syndrome.Endocrinol Metab Clin North Am. 2000 Mar;29(1):43-56. doi: 10.1016/s0889-8529(05)70115-8. Endocrinol Metab Clin North Am. 2000. PMID: 10732263 Review.
-
Hypothalamic-pituitary-adrenal axis recovery after treatment of Cushing's syndrome.J Neuroendocrinol. 2022 Aug;34(8):e13172. doi: 10.1111/jne.13172. Epub 2022 Jun 20. J Neuroendocrinol. 2022. PMID: 35726348 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

