Associations Between Surrogate Markers and Clinical Outcomes for Nononcologic Chronic Disease Treatments
- PMID: 38648042
- PMCID: PMC11036312
- DOI: 10.1001/jama.2024.4175
Associations Between Surrogate Markers and Clinical Outcomes for Nononcologic Chronic Disease Treatments
Abstract
Importance: Surrogate markers are increasingly used as primary end points in clinical trials supporting drug approvals.
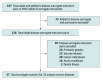
Objective: To systematically summarize the evidence from meta-analyses, systematic reviews and meta-analyses, and pooled analyses (hereafter, meta-analyses) of clinical trials examining the strength of association between treatment effects measured using surrogate markers and clinical outcomes in nononcologic chronic diseases.
Data sources: The Food and Drug Administration (FDA) Adult Surrogate Endpoint Table and MEDLINE from inception to March 19, 2023.
Study selection: Three reviewers selected meta-analyses of clinical trials; meta-analyses of observational studies were excluded.
Data extraction and synthesis: Two reviewers extracted correlation coefficients, coefficients of determination, slopes, effect estimates, or results from meta-regression analyses between surrogate markers and clinical outcomes.
Main outcomes and measures: Correlation coefficient or coefficient of determination, when reported, was classified as high strength (r ≥ 0.85 or R2 ≥ 0.72); primary findings were otherwise summarized.
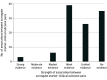
Results: Thirty-seven surrogate markers listed in FDA's table and used as primary end points in clinical trials across 32 unique nononcologic chronic diseases were included. For 22 (59%) surrogate markers (21 chronic diseases), no eligible meta-analysis was identified. For 15 (41%) surrogate markers (14 chronic diseases), at least 1 meta-analysis was identified, 54 in total (median per surrogate marker, 2.5; IQR, 1.3-6.0); among these, median number of trials and patients meta-analyzed was 18.5 (IQR, 12.0-43.0) and 90 056 (IQR, 20 109-170 014), respectively. The 54 meta-analyses reported 109 unique surrogate marker-clinical outcome pairs: 59 (54%) reported at least 1 r or R2, 10 (17%) of which reported at least 1 classified as high strength, whereas 50 (46%) reported slopes, effect estimates, or results of meta-regression analyses only, 26 (52%) of which reported at least 1 statistically significant result.
Conclusions and relevance: Most surrogate markers used as primary end points in clinical trials to support FDA approval of drugs treating nononcologic chronic diseases lacked high-strength evidence of associations with clinical outcomes from published meta-analyses.
Conflict of interest statement
Figures

Comment in
-
Surrogate Markers and Clinical Outcomes.JAMA. 2024 Sep 17;332(11):934-935. doi: 10.1001/jama.2024.14276. JAMA. 2024. PMID: 39167380 No abstract available.
-
Surrogate Markers and Clinical Outcomes.JAMA. 2024 Sep 17;332(11):935-936. doi: 10.1001/jama.2024.14273. JAMA. 2024. PMID: 39167387 No abstract available.
References
-
- US Food and Drug Administration . Surrogate endpoint resources for drug and biologic development. Accessed January 23, 2023. https://www.fda.gov/drugs/development-resources/surrogate-endpoint-resou...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

