Breast Milk Enema and Meconium Evacuation Among Preterm Infants: A Randomized Clinical Trial
- PMID: 38648060
- PMCID: PMC11981638
- DOI: 10.1001/jamanetworkopen.2024.7145
Breast Milk Enema and Meconium Evacuation Among Preterm Infants: A Randomized Clinical Trial
Abstract
Importance: Delayed meconium evacuation and delayed achievement of full enteral feeding among premature infants are associated with poor short- and long-term outcomes. Identifying a more effective and safer enema for meconium evacuation is imperative for improving neonatal care.
Objective: To examine whether breast milk enemas can shorten the time to complete meconium evacuation and achievement of full enteral feeding for preterm infants.
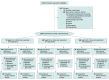
Design, setting, and participants: This randomized, open-label, parallel-group, single-center clinical trial was conducted from September 1, 2019, to September 30, 2022, among 286 preterm infants with a gestational age of 23 to 30 weeks in the neonatal ward of the Shengjing Hospital of China Medical University in Shenyang.
Interventions: Preterm infants were randomly assigned to receive either breast milk enemas or normal saline enemas 48 hours after birth.
Main outcome and measures: The primary outcomes were time to complete meconium evacuation and time to achieve full enteral feeding. Secondary outcomes were duration of hospitalization, weight at discharge, and duration of total parenteral nutrition. Intention-to-treat and per-protocol analyses were conducted.
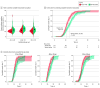
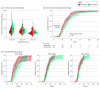
Results: In total, 286 preterm infants (mean [SD] gestational age, 198.8 [7.9] days; 166 boys [58.0%]) were eligible and included in this study. A total of 145 infants were randomized to the normal saline group, and 141 were randomized to the breast milk group. The time to achieve complete meconium evacuation was significantly shorter in the breast milk group than in the normal saline group (-2.2 days; 95% CI, -3.2 to -1.2 days). The time to achieve full enteral feeding was also significantly shorter in the breast milk group than in the normal saline group (-4.6 days; 95% CI, -8.0 to -1.2 days). The duration of total parenteral nutrition was significantly shorter in the breast milk group than in the normal saline group (-4.6 days; 95% CI, -8.6 to -1.0 days). There were no clinically notable differences in any other secondary or safety outcomes between the 2 groups.
Conclusions and relevance: In this randomized clinical trial testing the effects of breast milk enema on meconium evacuation, breast milk reduced the time to achieve complete meconium evacuation and achieve full enteral feeding for preterm infants with a gestational age of 23 to 30 weeks. Subgroup analyses highlight the need for tailored interventions based on gestational age considerations.
Trial registration: isrctn.org Identifier: ISRCTN17847514.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

