Kinesin Facilitates Phenotypic Targeting of Therapeutic Resistance in Advanced Prostate Cancer
- PMID: 38648082
- PMCID: PMC11296928
- DOI: 10.1158/1541-7786.MCR-23-1047
Kinesin Facilitates Phenotypic Targeting of Therapeutic Resistance in Advanced Prostate Cancer
Abstract
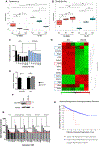
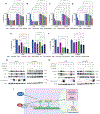
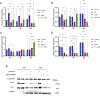
Understanding the mechanisms underlying resistance is critical to improving therapeutic outcomes in patients with metastatic castration-resistant prostate cancer. Previous work showed that dynamic interconversions between epithelial-mesenchymal transition to mesenchymal-epithelial transition defines the phenotypic landscape of prostate tumors, as a potential driver of the emergence of therapeutic resistance. In this study, we use in vitro and in vivo preclinical MDA PCa patient-derived xenograft models of resistant human prostate cancer to determine molecular mechanisms of cross-resistance between antiandrogen therapy and taxane chemotherapy, underlying the therapeutically resistant phenotype. Transcriptomic profiling revealed that resistant and sensitive prostate cancer C4-2B cells have a unique differential gene signature response to cabazitaxel. Gene pathway analysis showed that sensitive cells exhibit an increase in DNA damage, while resistant cells express genes associated with protein regulation in response to cabazitaxel. The patient-derived xenograft model specimens are from patients who have metastatic lethal castration-resistant prostate cancer, treated with androgen deprivation therapy, antiandrogens, and chemotherapy including second-line taxane chemotherapy, cabazitaxel. Immunohistochemistry revealed high expression of E-cadherin and low expression of vimentin resulting in redifferentiation toward an epithelial phenotype. Furthermore, the mitotic kinesin-related protein involved in microtubule binding and the SLCO1B3 transporter (implicated in cabazitaxel intracellular transport) are associated with resistance in these prostate tumors. Combinational targeting of kinesins (ispinesib) with cabazitaxel was more effective than single monotherapies in inducing cell death in resistant prostate tumors. Implications: Our findings are of translational significance in identifying kinesin as a novel target of cross-resistance toward enhancing therapeutic vulnerability and improved clinical outcomes in patients with advanced prostate cancer.
©2024 American Association for Cancer Research.
Conflict of interest statement
Figures




Similar articles
-
Androgens modify therapeutic response to cabazitaxel in models of advanced prostate cancer.Prostate. 2020 Sep;80(12):926-937. doi: 10.1002/pros.24015. Epub 2020 Jun 16. Prostate. 2020. PMID: 32542812 Free PMC article.
-
Multinucleation and Mesenchymal-to-Epithelial Transition Alleviate Resistance to Combined Cabazitaxel and Antiandrogen Therapy in Advanced Prostate Cancer.Cancer Res. 2016 Feb 15;76(4):912-26. doi: 10.1158/0008-5472.CAN-15-2078. Epub 2015 Dec 8. Cancer Res. 2016. PMID: 26645563 Free PMC article.
-
Exploitation of the Androgen Receptor to Overcome Taxane Resistance in Advanced Prostate Cancer.Adv Cancer Res. 2015;127:123-58. doi: 10.1016/bs.acr.2015.03.001. Epub 2015 Mar 29. Adv Cancer Res. 2015. PMID: 26093899 Review.
-
Marked response to cabazitaxel in prostate cancer xenografts expressing androgen receptor variant 7 and reversion of acquired resistance by anti-androgens.Prostate. 2020 Feb;80(2):214-224. doi: 10.1002/pros.23935. Epub 2019 Dec 4. Prostate. 2020. PMID: 31799745 Free PMC article.
-
Non-Coding RNAs Set a New Phenotypic Frontier in Prostate Cancer Metastasis and Resistance.Int J Mol Sci. 2021 Feb 20;22(4):2100. doi: 10.3390/ijms22042100. Int J Mol Sci. 2021. PMID: 33672595 Free PMC article. Review.
Cited by
-
Impact of cell plasticity on prostate tumor heterogeneity and therapeutic response.Am J Clin Exp Urol. 2024 Dec 15;12(6):331-351. doi: 10.62347/YFRP8901. eCollection 2024. Am J Clin Exp Urol. 2024. PMID: 39839748 Free PMC article. Review.
References
-
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. 2023;73(1):17–48. - PubMed
-
- Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. The New England journal of medicine. 2012;367(13):1187–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

