Beyond the VSG layer: Exploring the role of intrinsic disorder in the invariant surface glycoproteins of African trypanosomes
- PMID: 38648216
- PMCID: PMC11065263
- DOI: 10.1371/journal.ppat.1012186
Beyond the VSG layer: Exploring the role of intrinsic disorder in the invariant surface glycoproteins of African trypanosomes
Abstract
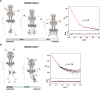
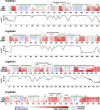
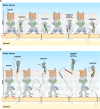
In the bloodstream of mammalian hosts, African trypanosomes face the challenge of protecting their invariant surface receptors from immune detection. This crucial role is fulfilled by a dense, glycosylated protein layer composed of variant surface glycoproteins (VSGs), which undergo antigenic variation and provide a physical barrier that shields the underlying invariant surface glycoproteins (ISGs). The protective shield's limited permeability comes at the cost of restricted access to the extracellular host environment, raising questions regarding the specific function of the ISG repertoire. In this study, we employ an integrative structural biology approach to show that intrinsically disordered membrane-proximal regions are a common feature of members of the ISG super-family, conferring the ability to switch between compact and elongated conformers. While the folded, membrane-distal ectodomain is buried within the VSG layer for compact conformers, their elongated counterparts would enable the extension beyond it. This dynamic behavior enables ISGs to maintain a low immunogenic footprint while still allowing them to engage with the host environment when necessary. Our findings add further evidence to a dynamic molecular organization of trypanosome surface antigens wherein intrinsic disorder underpins the characteristics of a highly flexible ISG proteome to circumvent the constraints imposed by the VSG coat.
Copyright: © 2024 Sülzen et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Antigenic variation in trypanosomes: enhanced phenotypic variation in a eukaryotic parasite.Adv Parasitol. 2001;49:1-70. doi: 10.1016/s0065-308x(01)49037-3. Adv Parasitol. 2001. PMID: 11461029 Review.
-
Evolution of the variant surface glycoprotein family in African trypanosomes.Trends Parasitol. 2022 Jan;38(1):23-36. doi: 10.1016/j.pt.2021.07.012. Epub 2021 Aug 7. Trends Parasitol. 2022. PMID: 34376326 Review.
-
Bloodstream form Trypanosome plasma membrane proteins: antigenic variation and invariant antigens.Parasitology. 2010 Dec;137(14):2029-39. doi: 10.1017/S0031182009992034. Epub 2010 Jan 29. Parasitology. 2010. PMID: 20109254 Review.
-
A Host-Pathogen Interaction Reduced to First Principles: Antigenic Variation in T. brucei.Results Probl Cell Differ. 2015;57:23-46. doi: 10.1007/978-3-319-20819-0_2. Results Probl Cell Differ. 2015. PMID: 26537376 Review.
-
How Does the VSG Coat of Bloodstream Form African Trypanosomes Interact with External Proteins?PLoS Pathog. 2015 Dec 31;11(12):e1005259. doi: 10.1371/journal.ppat.1005259. eCollection 2015 Dec. PLoS Pathog. 2015. PMID: 26719972 Free PMC article. Review.
Cited by
-
Small-angle X-ray scattering profile calculation for high-resolution models of biomacromolecules.J Appl Crystallogr. 2025 Jul 16;58(Pt 4):1332-1346. doi: 10.1107/S160057672500562X. eCollection 2025 Aug 1. J Appl Crystallogr. 2025. PMID: 40765969 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

