Highly Active W2C-Based Composites for the HER in Alkaline Solution: the Role of Surface Oxide Species
- PMID: 38648335
- PMCID: PMC11071040
- DOI: 10.1021/acsami.4c01612
Highly Active W2C-Based Composites for the HER in Alkaline Solution: the Role of Surface Oxide Species
Abstract
The hydrogen evolution reaction (HER) is a crucial electrochemical process for the proposed hydrogen economy since it has the potential to provide pure hydrogen for fuel cells. Nowadays, hydrogen electroproduction is considerably expensive, so promoting the development of new non-noble catalysts for the cathode of alkaline electrolyzers appears as a suitable way to reduce the costs of this technology. In this sense, a series of tungsten-based carbide materials have been synthesized by the urea-glass route as candidates to improve the HER in alkaline media. Moreover, two different pyridinium-based ionic liquids were employed to modify the surface of the carbide grains and control the amount and nature of their surface species. The main results indicate that the catalyst surface composition is modified in the hybrid materials, which are then distinguished by the appearance of tungsten suboxide structures. This implies the action of ionic liquids as reducing agents. Consequently, differential electrochemical mass spectrometry (DEMS) is used to precisely determine the onset potentials and rate-determining steps (RDS) for the HER in alkaline media. Remarkably, the modified surfaces show high catalytic performance (overpotentials between 45 and 60 mV) and RDS changes from Heyrovsky-Volmer to Heyrovsky as the surface oxide structures get reduced. H2O molecule reduction is then faster at tungsten suboxide, which allows the formation of the adsorbed hydrogen at the surface, boosting the catalytic activity and the kinetics of the alkaline HER.
Keywords: AEMWE; HER; ionic liquid; suboxide; tungsten carbide.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
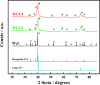
 , 00-035-0776; magenta) from
the PDF2 database are also depicted for comparison purposes. The signals
related to WC traces in the commercial carbide are marked with
, 00-035-0776; magenta) from
the PDF2 database are also depicted for comparison purposes. The signals
related to WC traces in the commercial carbide are marked with  .
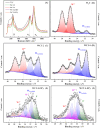
.

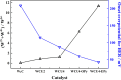
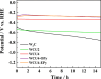
References
-
- Abe J. O.; Popoola A. P. I.; Ajenifuja E.; Popoola O. M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrogen Energy 2019, 44 (29), 15072–15086. 10.1016/j.ijhydene.2019.04.068. - DOI
-
- Manabe A.; Kashiwase M.; Hashimoto T.; Hayashida T.; Kato A.; Hirao K.; Shimomura I.; Nagashima I. Basic study of alkaline water electrolysis. Electrochim. Acta 2013, 100, 249–256. 10.1016/j.electacta.2012.12.105. - DOI
-
- Schmidt O.; Gambhir A.; Staffell I.; Hawkes A.; Nelson J.; Few S. Future cost and performance of water electrolysis: An expert elicitation study. Int. J. Hydrogen Energy 2017, 42 (52), 30470–30492. 10.1016/j.ijhydene.2017.10.045. - DOI
-
- de Groot M. T.; Kraakman J.; Garcia Barros R. L. Optimal operating parameters for advanced alkaline water electrolysis. Int. J. Hydrogen Energy 2022, 47 (82), 34773–34783. 10.1016/j.ijhydene.2022.08.075. - DOI
-
- Vermeiren P.; Adriansens W.; Leysen R. Zirfon®: A new separator for Ni-H2 batteries and alkaline fuel cells. Int. J. Hydrogen Energy 1996, 21 (8), 679–684. 10.1016/0360-3199(95)00132-8. - DOI
LinkOut - more resources
Full Text Sources

