Discovery and Structure-Activity Relationships of 2,5-Dimethoxyphenylpiperidines as Selective Serotonin 5-HT2A Receptor Agonists
- PMID: 38648420
- PMCID: PMC11089506
- DOI: 10.1021/acs.jmedchem.4c00082
Discovery and Structure-Activity Relationships of 2,5-Dimethoxyphenylpiperidines as Selective Serotonin 5-HT2A Receptor Agonists
Abstract
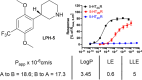
Classical psychedelics such as psilocybin, lysergic acid diethylamide (LSD), and N,N-dimethyltryptamine (DMT) are showing promising results in clinical trials for a range of psychiatric indications, including depression, anxiety, and substance abuse disorder. These compounds are characterized by broad pharmacological activity profiles, and while the acute mind-altering effects can be ascribed to their shared agonist activity at the serotonin 2A receptor (5-HT2AR), their apparent persistent therapeutic effects are yet to be decidedly linked to activity at this receptor. We report herein the discovery of 2,5-dimethoxyphenylpiperidines as a novel class of selective 5-HT2AR agonists and detail the structure-activity investigations leading to the identification of LPH-5 [analogue (S)-11] as a selective 5-HT2AR agonist with desirable drug-like properties.
Conflict of interest statement
The authors declare the following competing financial interest(s): E.M.R., A.A.J. and J.L.K. are cofounders of and hold equity in Lophora, a startup company pursuing the compound class detailed in this manuscript.
Figures
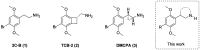
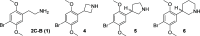


Similar articles
-
The neuropharmacology of sleep paralysis hallucinations: serotonin 2A activation and a novel therapeutic drug.Psychopharmacology (Berl). 2018 Nov;235(11):3083-3091. doi: 10.1007/s00213-018-5042-1. Epub 2018 Oct 5. Psychopharmacology (Berl). 2018. PMID: 30288594 Free PMC article. Review.
-
Hallucinogenic 5-HT2AR agonists LSD and DOI enhance dopamine D2R protomer recognition and signaling of D2-5-HT2A heteroreceptor complexes.Biochem Biophys Res Commun. 2014 Jan 3;443(1):278-84. doi: 10.1016/j.bbrc.2013.11.104. Epub 2013 Dec 2. Biochem Biophys Res Commun. 2014. PMID: 24309097
-
Discovery of β-Arrestin-Biased 25CN-NBOH-Derived 5-HT2A Receptor Agonists.J Med Chem. 2022 Sep 22;65(18):12031-12043. doi: 10.1021/acs.jmedchem.2c00702. Epub 2022 Sep 13. J Med Chem. 2022. PMID: 36099411 Free PMC article.
-
The selective 5-HT2A receptor agonist 25CN-NBOH: Structure-activity relationship, in vivo pharmacology, and in vitro and ex vivo binding characteristics of [3H]25CN-NBOH.Biochem Pharmacol. 2020 Jul;177:113979. doi: 10.1016/j.bcp.2020.113979. Epub 2020 Apr 13. Biochem Pharmacol. 2020. PMID: 32298690
-
Serotonergic Psychedelics: A Comparative Review of Efficacy, Safety, Pharmacokinetics, and Binding Profile.Biol Psychiatry Cogn Neurosci Neuroimaging. 2024 May;9(5):472-489. doi: 10.1016/j.bpsc.2024.01.007. Epub 2024 Feb 1. Biol Psychiatry Cogn Neurosci Neuroimaging. 2024. PMID: 38301886 Review.
Cited by
-
The Selective Serotonin 5‑HT2A Receptor Agonist (S)‑3-(2,5-Dimethoxy-4-(trifluoromethyl)phenyl)piperidine (LPH-5) Induces Persistent and Robust Antidepressant-Like Effects in Rodents.ACS Pharmacol Transl Sci. 2025 May 29;8(6):1791-1803. doi: 10.1021/acsptsci.5c00208. eCollection 2025 Jun 13. ACS Pharmacol Transl Sci. 2025. PMID: 40534669
-
The Selective 5HT2A Receptor Agonist, 25CN-NBOH Exerts Excitatory and Inhibitory Cellular Actions on Mouse Medial Prefrontal Cortical Neurons.Synapse. 2025 Mar;79(2):e70014. doi: 10.1002/syn.70014. Synapse. 2025. PMID: 40128102 Free PMC article.
References
-
- Carhart-Harris R. L.; Bolstridge M.; Day C. M. J.; Rucker J.; Watts R.; Erritzoe D. E.; Kaelen M.; Giribaldi B.; Bloomfield M.; Pilling S.; et al. Psilocybin with psychological support for treatment-resistant depression: six-month follow-up. Psychopharmacology 2018, 235 (2), 399–408. 10.1007/s00213-017-4771-x. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

