Discovery and Structure-Activity Relationships of 2,5-Dimethoxyphenylpiperidines as Selective Serotonin 5-HT2A Receptor Agonists
- PMID: 38648420
- PMCID: PMC11089506
- DOI: 10.1021/acs.jmedchem.4c00082
Discovery and Structure-Activity Relationships of 2,5-Dimethoxyphenylpiperidines as Selective Serotonin 5-HT2A Receptor Agonists
Abstract
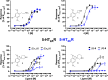
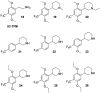
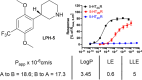
Classical psychedelics such as psilocybin, lysergic acid diethylamide (LSD), and N,N-dimethyltryptamine (DMT) are showing promising results in clinical trials for a range of psychiatric indications, including depression, anxiety, and substance abuse disorder. These compounds are characterized by broad pharmacological activity profiles, and while the acute mind-altering effects can be ascribed to their shared agonist activity at the serotonin 2A receptor (5-HT2AR), their apparent persistent therapeutic effects are yet to be decidedly linked to activity at this receptor. We report herein the discovery of 2,5-dimethoxyphenylpiperidines as a novel class of selective 5-HT2AR agonists and detail the structure-activity investigations leading to the identification of LPH-5 [analogue (S)-11] as a selective 5-HT2AR agonist with desirable drug-like properties.
Conflict of interest statement
The authors declare the following competing financial interest(s): E.M.R., A.A.J. and J.L.K. are cofounders of and hold equity in Lophora, a startup company pursuing the compound class detailed in this manuscript.
Figures
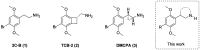
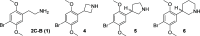


References
-
- Carhart-Harris R. L.; Bolstridge M.; Day C. M. J.; Rucker J.; Watts R.; Erritzoe D. E.; Kaelen M.; Giribaldi B.; Bloomfield M.; Pilling S.; et al. Psilocybin with psychological support for treatment-resistant depression: six-month follow-up. Psychopharmacology 2018, 235 (2), 399–408. 10.1007/s00213-017-4771-x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

