Single-cell transcriptomics unveiled that early life BDE-99 exposure reprogrammed the gut-liver axis to promote a proinflammatory metabolic signature in male mice at late adulthood
- PMID: 38648751
- PMCID: PMC11199921
- DOI: 10.1093/toxsci/kfae047
Single-cell transcriptomics unveiled that early life BDE-99 exposure reprogrammed the gut-liver axis to promote a proinflammatory metabolic signature in male mice at late adulthood
Abstract
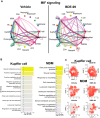
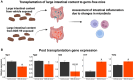
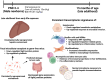
Polybrominated diphenyl ethers (PBDEs) are legacy flame retardants that bioaccumulate in the environment. The gut microbiome is an important regulator of liver functions including xenobiotic biotransformation and immune regulation. We recently showed that neonatal exposure to polybrominated diphenyl ether-99 (BDE-99), a human breast milk-enriched PBDE congener, up-regulated proinflammation-related and down-regulated drug metabolism-related genes predominantly in males in young adulthood. However, the persistence of this dysregulation into late adulthood, differential impact among hepatic cell types, and the involvement of the gut microbiome from neonatal BDE-99 exposure remain unknown. To address these knowledge gaps, male C57BL/6 mouse pups were orally exposed to corn oil (10 ml/kg) or BDE-99 (57 mg/kg) once daily from postnatal days 2-4. At 15 months of age, neonatal BDE-99 exposure down-regulated xenobiotic and lipid-metabolizing enzymes and up-regulated genes involved in microbial influx in hepatocytes. Neonatal BDE-99 exposure also increased the hepatic proportion of neutrophils and led to a predicted increase of macrophage migration inhibitory factor signaling. This was associated with decreased intestinal tight junction protein (Tjp) transcripts, altered gut environment, and dysregulation of inflammation-related metabolites. ScRNA-seq using germ-free (GF) mice demonstrated the necessity of a normal gut microbiome in maintaining hepatic immune tolerance. Microbiota transplant to GF mice using large intestinal microbiome from adults neonatally exposed to BDE-99 down-regulated Tjp transcripts and up-regulated several cytokines in large intestine. In conclusion, neonatal BDE-99 exposure reprogrammed cell type-specific gene expression and cell-cell communication in liver towards proinflammation, and this may be partly due to the dysregulated gut environment.
Keywords: RNA-seq; environmental chemicals; microbiome; omics research; persistent organic chemicals; systems biology.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Society of Toxicology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures







Similar articles
-
Regulation of protein-coding gene and long noncoding RNA pairs in liver of conventional and germ-free mice following oral PBDE exposure.PLoS One. 2018 Aug 1;13(8):e0201387. doi: 10.1371/journal.pone.0201387. eCollection 2018. PLoS One. 2018. PMID: 30067809 Free PMC article.
-
Novel Interactions between Gut Microbiome and Host Drug-Processing Genes Modify the Hepatic Metabolism of the Environmental Chemicals Polybrominated Diphenyl Ethers.Drug Metab Dispos. 2017 Nov;45(11):1197-1214. doi: 10.1124/dmd.117.077024. Epub 2017 Sep 1. Drug Metab Dispos. 2017. PMID: 28864748 Free PMC article.
-
Neonatal Exposure to BPA, BDE-99, and PCB Produces Persistent Changes in Hepatic Transcriptome Associated With Gut Dysbiosis in Adult Mouse Livers.Toxicol Sci. 2021 Oct 27;184(1):83-103. doi: 10.1093/toxsci/kfab104. Toxicol Sci. 2021. PMID: 34453844 Free PMC article.
-
Polybrominated diphenyl ethers: human tissue levels and toxicology.Rev Environ Contam Toxicol. 2004;183:55-97. doi: 10.1007/978-1-4419-9100-3_3. Rev Environ Contam Toxicol. 2004. PMID: 15369322 Review.
-
Distribution of polybrominated diphenyl ethers in breast milk, cord blood and placentas: a systematic review.Environ Sci Pollut Res Int. 2017 Sep;24(27):21548-21573. doi: 10.1007/s11356-017-9821-8. Epub 2017 Aug 22. Environ Sci Pollut Res Int. 2017. PMID: 28831660
Cited by
-
Environmental Pollutants, Occupational Exposures, and Liver Disease.Semin Liver Dis. 2025 Jun;45(2):148-166. doi: 10.1055/a-2540-2861. Epub 2025 Mar 21. Semin Liver Dis. 2025. PMID: 40118102 Free PMC article. Review.
-
Single-cell transcriptomics showed that maternal PCB exposure dysregulated ER stress-mediated cell type-specific responses in the liver of female offspring.bioRxiv [Preprint]. 2025 Jun 8:2025.06.04.657944. doi: 10.1101/2025.06.04.657944. bioRxiv. 2025. PMID: 40501930 Free PMC article. Preprint.
References
-
- Abdelhamid M. K., Rychlik I., Hess C., Hatfaludi T., Crhanova M., Karasova D., Lagler J., Liebhart D., Hess M., Paudel S. (2021). Typhlitis induced by histomonas meleagridis affects relative but not the absolute Escherichia coli counts and invasion in the gut in turkeys. Vet. Res. 52, 92. - PMC - PubMed
-
- Alexa, A., and Rahnenfuhrer, J. (2017). topGO. Bioconductor. 10.18129/B9.BIOC.TOPGO. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

