Cadherin-11 targeted cell-specific liposomes enabled skin fibrosis treatment by inducing apoptosis
- PMID: 38648957
- PMCID: PMC11705614
- DOI: 10.1016/j.jconrel.2024.04.029
Cadherin-11 targeted cell-specific liposomes enabled skin fibrosis treatment by inducing apoptosis
Abstract
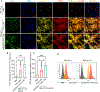
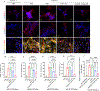
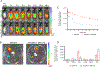
Continuous and aberrant activation of myofibroblasts is the hallmark of pathological fibrosis (e.g., abnormal wound healing). The deposition of excessive extracellular matrix (ECM) components alters or increases the stiffness of tissue and primarily accounts for multiple organ dysfunctions. Among various proteins, Cadherin-11 (CDH11) has been reported to be overexpressed on myofibroblasts in fibrotic tissues. Anti-apoptotic proteins such as (B cell lymphoma-2) (BCL-2) are also upregulated on myofibroblasts. Therefore, we hypothesize that CDH11 could be a targeted domain for cell-specific drug delivery and targeted inhibition of BCL-2 to ameliorate the development of fibrosis in the skin. To prove our hypothesis, we have developed liposomes (LPS) conjugated with CDH11 neutralizing antibody (antiCDH11) to target cell surface CDH11 and loaded these LPS with a BCL-2 inhibitor, Navitoclax (NAVI), to induce apoptosis of CDH11 expressing fibroblasts. The developed LPS were evaluated for physicochemical characterization, stability, in vitro therapeutic efficacy using dermal fibroblasts, and in vivo therapeutic efficacy in bleomycin-induced skin fibrosis model in mice. The findings from in vitro and in vivo studies confirmed that selectivity of LPS was improved towards CDH11 expressing myofibroblasts, thereby improving therapeutic efficacy with no indication of adverse effects. Hence, this novel research work represents a versatile LPS strategy that exhibits promising potential for treating skin fibrosis.
Keywords: Cadherin 11; Dermal fibrosis; Lipid nanoparticle; Myofibroblast; Scleroderma.
Copyright © 2024 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors report no conflict of interest.
Figures

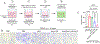

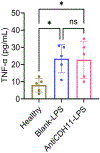
References
-
- Pathogenesis of Systemic Sclerosis: Recent Insights of Molecular and Cellular Mechanisms and Therapeutic Opportunities - John Varga, Maria Trojanowska, Masataka Kuwana, 2017, (n.d.). 10.5301/jsrd.5000249 (accessed November 29, 2023). - DOI
-
- Emerging targets of disease-modifying therapy for systemic sclerosis Nat. Rev. Rheumatol., (n.d.). https://www.nature.com/articles/s41584-019-0184-z (accessed November 29, 2023). - PubMed
-
- Scleroderma Facts, n.d. https://scleroderma.org/wp-content/uploads/2022/02/SIP-2022-Updated-7_5_....
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

