Daratumumab-based quadruplet therapy for transplant-eligible newly diagnosed multiple myeloma with high cytogenetic risk
- PMID: 38649340
- PMCID: PMC11035596
- DOI: 10.1038/s41408-024-01030-w
Daratumumab-based quadruplet therapy for transplant-eligible newly diagnosed multiple myeloma with high cytogenetic risk
Abstract
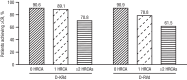
In the MASTER study (NCT03224507), daratumumab+carfilzomib/lenalidomide/dexamethasone (D-KRd) demonstrated promising efficacy in transplant-eligible newly diagnosed multiple myeloma (NDMM). In GRIFFIN (NCT02874742), daratumumab+lenalidomide/bortezomib/dexamethasone (D-RVd) improved outcomes for transplant-eligible NDMM. Here, we present a post hoc analysis of patients with high-risk cytogenetic abnormalities (HRCAs; del[17p], t[4;14], t[14;16], t[14;20], or gain/amp[1q21]). Among 123 D-KRd patients, 43.1%, 37.4%, and 19.5% had 0, 1, or ≥2 HRCAs. Among 120 D-RVd patients, 55.8%, 28.3%, and 10.8% had 0, 1, or ≥2 HRCAs. Rates of complete response or better (best on study) for 0, 1, or ≥2 HRCAs were 90.6%, 89.1%, and 70.8% for D-KRd, and 90.9%, 78.8%, and 61.5% for D-RVd. At median follow-up (MASTER, 31.1 months; GRIFFIN, 49.6 months for randomized patients/59.5 months for safety run-in patients), MRD-negativity rates as assessed by next-generation sequencing (10-5) were 80.0%, 86.4%, and 83.3% for 0, 1, or ≥2 HRCAs for D-KRd, and 76.1%, 55.9%, and 61.5% for D-RVd. PFS was similar between studies and superior for 0 or 1 versus ≥2 HRCAs: 36-month PFS rates for D-KRd were 89.9%, 86.2%, and 52.4%, and 96.7%, 90.5%, and 53.5% for D-RVd. These data support the use of daratumumab-containing regimens for transplant-eligible NDMM with HCRAs; however, additional strategies are needed for ultra-high-risk disease (≥2 HRCAs). Video Abstract.
© 2024. The Author(s).
Conflict of interest statement
RS served as a consultant or in an advisory role for Sanofi-Aventis, Janssen Oncology, and Oncopeptides; and received research funding from Sanofi. JLK consulted for AbbVie, Janssen, Roche/Genentech, Bristol Myers Squibb, Celgene, and Tecnopharma; received research funding from AbbVie, Amgen, Bristol Myers Squibb, Fortis Therapeutics, Heidelberg Pharma, Janssen, Novartis, Roche/Genentech, Sutro Biopharma, and Takeda; received honoraria from AbbVie, Janssen, Roche/Genentech, and Tecnopharma; and holds a membership on a board or advisory committee for Incyte and TG Therapeutics. JL received honoraria from Great Debates & Updates – Hematologic Malignancies. TMS received consulting fees from BioLineRx, Janssen, and Sanofi; and received support for attending meetings and/or travel from Sanofi. DWS consulted for, holds membership on an entity’s board of directors for, or served on advisory committees for Arcellx, GSK, and Janssen; and consulted for Bristol Myers Squibb, Pfizer, AbbVie, and Sanofi. BR received honoraria from Incyte, Bristol Myers Squibb, and PharmaEssentia. BD received consulting fees from Janssen, Sanofi, Genentech, and AbbVie; received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Sanofi, Janssen, Karyopharm Therapeutics, Bristol Myers Squibb, and GSK; and participated on a data safety monitoring board or advisory board for Amgen, Takeda, Janssen, Sanofi, Arcellx, and Natera. CR consulted for Janssen, Bristol Myers Squibb, Takeda, AbbVie, Karyopharm Therapeutics, and Artiva; and served on a speakers bureau for Janssen, Bristol Myers Squibb, Takeda, AbbVie, Karyopharm Therapeutics, and Artiva. SC received research funding from Janssen, AbbVie, C4 Therapeutics, Takeda, and CARSgen Therapeutics; and received honoraria from Janssen, Sanofi, and GSK. SB received grants or contracts from an entity from the Amyloidosis Foundation; and received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Adaptive Biotechnologies. LDA holds a membership on an entity’s board of directors or advisory committees for, served as a consultant for, and received honoraria from GSK, Bristol Myers Squibb, Celgene, Janssen, Amgen, Oncopeptides, Karyopharm Therapeutics, AbbVie, BeiGene, Sanofi, and Cellectar Biosciences. BRD received research funding from Pfizer, Janssen, Takeda, MEI, Angiocrine, and Poseida; and served on a speakers bureau for Jazz Pharmaceuticals and Celgene. PH served as a consultant for and received honoraria from Sanofi, Amgen, Janssen, Karyopharm Therapeutics, Takeda, and Kite; was a consultant for and received honoraria and research funding from Bristol Myers Squibb; received research funding from Millennium and Spectrum Pharmaceuticals; received honoraria from Novartis, Incyte, GSK, and AbbVie; and was a consultant for Pharmacyclics. NS received research funding from Bristol Myers Squibb/Celgene, Janssen, bluebird bio, Sutro Biopharma, TeneoBio, Poseida, Nektar, and Precision Biosciences; served as a consultant for GSK, Amgen, Indapta Therapeutics, Sanofi, CareDx, Kite, Karyopharm Therapeutics, Oncopeptides, and CSL Behring; and is a current employee and equity holder of AstraZeneca. NB served on a speakers bureau for Amgen, Sanofi, and Genzyme; and served on an advisory board for Sanofi, Genzyme, and Janssen. SAH served as a consultant for Bristol Myers Squibb/Celgene, Janssen, Takeda, Pfizer, Oncopeptides, GSK, Secura Bio, and Sanofi; and received research funding from Bristol Myers Squibb and Oncopeptides. CC received honoraria from Takeda, Bristol Myers Squibb, Pfizer, and Janssen. AJ served as a consultant or in an advisory role for and received honoraria from AbbVie, Amgen, Bristol Myers Squibb, Celgene, GSK, Janssen, Karyopharm Therapeutics, and Sanofi. TMW served as a consultant for Carevive, Janssen, Seagen, and Sanofi. RZO received research funding from Asylia Therapeutics, Biotheryx, Heidelberg Pharma, CARsgen Therapeutics, Bristol Myers Squibb/Celgene, Exelixis, Janssen Biotech, Sanofi-Aventis, and Takeda Pharmaceuticals North America; received honoraria from and holds a membership on an entity’s board of directors or advisory committees for AbbVie, Biotheryx, Bristol Myers Squibb, Janssen Biotech, Karyopharm Therapeutics, Meridian Therapeutics, Monte Rosa Therapeutics, Neoleukin Corporation, Oncopeptides AB, Regeneron Pharmaceuticals, Sanofi-Aventis, and Takeda Pharmaceuticals North America; and is a current stockholder of Asylia Therapeutics. KHS received honoraria from Bristol Myers Squibb, Janssen, GSK, Adaptive Biotechnologies, Sanofi, Takeda, and Amgen; served as an ad hoc member of an advisory committee for GSK, Janssen, and Bristol Myers Squibb; served on a speakers bureau for GSK, Bristol Myers Squibb, Sanofi, Karyopharm Therapeutics, Takeda, Janssen, Adaptive Biotechnologies, and Amgen; received research funding from AbbVie and Karyopharm Therapeutics; and is the principal investigator of clinical trials sponsored by Janssen and Bristol Myers Squibb, with all research outside the scope of the submitted work. AJC served as a consultant for and received research funding from Janssen, Bristol Myers Squibb, and AbbVie; received research funding from Harpoon, Sanofi-Aventis, and Nektar; was a consultant for Allogene, EUSA, GSK, and Secura Bio; and received research funding from and holds a membership on an entity’s board of directors or advisory committees for Adaptive Biotechnologies. SG received honoraria from Carevive and OncLive; and received research funding from Carevive and Pack Health. LJC served as a consultant or in an advisory role for AbbVie, Amgen, Celgene, Karyopharm Therapeutics, and Sanofi; served on a speakers bureau for Amgen and Sanofi; received honoraria from Amgen, Celgene, Janssen, Karyopharm Therapeutics, and Sanofi; and received research funding from Amgen and Janssen. SZU served as a consultant for Celgene, Amgen, Janssen Oncology, Seattle Genetics, Takeda, GSK, Karyopharm Therapeutics, AbbVie, SkylineDx, Merck, Oncopeptides, Genentech, Gilead Sciences, and Bristol Myers Squibb/Celgene; served on a speakers bureau for Takeda, Amgen, Janssen Oncology, Sanofi, and Bristol Myers Squibb/Celgene; and received research funding from Celgene and Array BioPharma. PGR received research funding from Oncopeptides, Celgene/Bristol Myers Squibb, Takeda, and Karyopharm Therapeutics; and served on advisory committees for Oncopeptides, Celgene/Bristol Myers Squibb, Takeda, Karyopharm Therapeutics, Janssen, Sanofi, Secura Bio, GSK, Regeneron Pharmaceuticals, AstraZeneca, and Protocol Intelligence. PMV served as a consultant for, received honoraria from, and holds a membership on an entity’s board of directors or advisory committees for AbbVie, Bristol Myers Squibb, Karyopharm Therapeutics, Regeneron Pharmaceuticals, and Sanofi. HP, A Cortoos, SP, and TSL are current equity holders and employees of Janssen. NSC, KNG, EM, A Chari, and NN have nothing to disclose.
Figures
References
-
- Lammerts van Bueren J, Jakobs D, Kaldenhoven N, Roza M, Hiddingh S, Meesters J, et al. Direct in vitro comparison of daratumumab with surrogate analogs of CD38 antibodies MOR03087, SAR650984 and Ab79. Blood. 2014;124:3474. doi: 10.1182/blood.V124.21.3474.3474. - DOI
-
- Overdijk MB, Verploegen S, Bögels M, van Egmond M, Lammerts van Bueren JJ, et al. Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. MAbs. 2015;7:311–21. doi: 10.1080/19420862.2015.1007813. - DOI - PMC - PubMed

