PD-L1- and IL-4-expressing basophils promote pathogenic accumulation of T follicular helper cells in lupus
- PMID: 38649353
- PMCID: PMC11035650
- DOI: 10.1038/s41467-024-47691-w
PD-L1- and IL-4-expressing basophils promote pathogenic accumulation of T follicular helper cells in lupus
Abstract
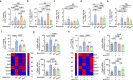
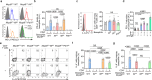
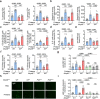
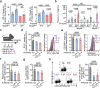
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by anti-nuclear autoantibodies whose production is promoted by autoreactive T follicular helper (TFH) cells. During SLE pathogenesis, basophils accumulate in secondary lymphoid organs (SLO), amplify autoantibody production and disease progression through mechanisms that remain to be defined. Here, we provide evidence for a direct functional relationship between TFH cells and basophils during lupus pathogenesis, both in humans and mice. PD-L1 upregulation on basophils and IL-4 production are associated with TFH and TFH2 cell expansions and with disease activity. Pathogenic TFH cell accumulation, maintenance, and function in SLO were dependent on PD-L1 and IL-4 in basophils, which induced a transcriptional program allowing TFH2 cell differentiation and function. Our study establishes a direct mechanistic link between basophils and TFH cells in SLE that promotes autoantibody production and lupus nephritis.
© 2024. The Author(s).
Conflict of interest statement
N.C. holds a patent related to compositions and methods for treating or preventing lupus (W020120710042). C.P. and N.C. are coinventors of the patent WO2016128565A1 related to the use of PTGDR-1 and PTGDR-2 antagonists for the prevention or treatment of systemic lupus erythematosus. No other disclosures relevant to this article are reported. The remaining authors declare no competing interests relevant to this article.
Figures




References
-
- Gensous N, Schmitt N, Richez C, Ueno H, Blanco P. T follicular helper cells, interleukin-21 and systemic lupus erythematosus. Rheumatology. 2017;56:516–523. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- ANR-19-CE17-0029 BALUMET/Agence Nationale de la Recherche (French National Research Agency)
- ANRPIA-10-LABX-0017 INFLAMEX/Agence Nationale de la Recherche (French National Research Agency)
- EQU201903007794/Fondation pour la Recherche Médicale (Foundation for Medical Research in France)
- UMR1149/Institut National de la Santé et de la Recherche Médicale (National Institute of Health and Medical Research)
- EMR8252/Centre National de la Recherche Scientifique (National Center for Scientific Research)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

