CROCuS, a Phase II Study Evaluating the Antiviral Activity, Clinical Outcomes, and Safety of Rilematovir in Children Aged ≥ 28 Days and ≤ 3 Years with Acute Respiratory Tract Infection Due to Respiratory Syncytial Virus
- PMID: 38649595
- PMCID: PMC11192697
- DOI: 10.1007/s40272-024-00625-x
CROCuS, a Phase II Study Evaluating the Antiviral Activity, Clinical Outcomes, and Safety of Rilematovir in Children Aged ≥ 28 Days and ≤ 3 Years with Acute Respiratory Tract Infection Due to Respiratory Syncytial Virus
Abstract
Background: Respiratory syncytial virus (RSV) causes significant morbidity and mortality in children aged ≤ 5 years and adults aged ≥ 60 years worldwide. Despite this, RSV-specific therapeutic options are limited. Rilematovir is an investigational, orally administered inhibitor of RSV fusion protein-mediated viral entry.
Objective: To establish the antiviral activity, clinical outcomes, safety, and tolerability of rilematovir (low or high dose) in children aged ≥ 28 days and ≤ 3 years with RSV disease.
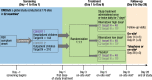
Methods: CROCuS was a multicenter, international, double-blind, placebo-controlled, randomized, adaptive phase II study, wherein children aged ≥ 28 days and ≤ 3 years with confirmed RSV infection who were either hospitalized (Cohort 1) or treated as outpatients (Cohort 2) were randomized (1:1:1) to receive rilematovir (low or high dose) or placebo. Study treatment was administered daily as an oral suspension from days 1 to 7, with dosing based on weight and age groups. The primary objective was to establish antiviral activity of rilematovir by evaluating the area under the plasma concentration-time curve of RSV viral load in nasal secretions from baseline through day 5. Severity and duration of RSV signs and symptoms and the safety and tolerability of rilematovir were also assessed through day 28 (± 3).
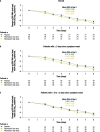
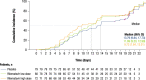
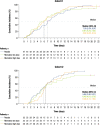
Results: In total, 246 patients were randomized, treated, and included in the safety analysis population (Cohort 1: 147; Cohort 2: 99). Of these, 231 were included in the intent-to-treat-infected analysis population (Cohort 1: 138; Cohort 2: 93). In both cohorts, demographics were generally similar across treatment groups. In both cohorts combined, the difference (95% confidence interval) in the mean area under the plasma concentration-time curve of RSV RNA viral load through day 5 was - 1.25 (- 2.672, 0.164) and - 1.23 (- 2.679, 0.227) log10 copies∙days/mL for the rilematovir low-dose group and the rilematovir high-dose group, respectively, when compared with placebo. The estimated Kaplan-Meier median (95% confidence interval) time to resolution of key RSV symptoms in the rilematovir low-dose, rilematovir high-dose, and placebo groups of Cohort 1 was 6.01 (4.24, 7.25), 5.82 (4.03, 8.18), and 7.05 (5.34, 8.97) days, respectively; in Cohort 2, estimates were 6.45 (4.81, 9.70), 6.26 (5.41, 7.84), and 5.85 (3.90, 8.27) days, respectively. A similar incidence of adverse events was reported in patients treated with rilematovir and placebo in Cohort 1 (rilematovir: 61.9%; placebo: 58.0%) and Cohort 2 (rilematovir: 50.8%; placebo: 47.1%), with most reported as grade 1 or 2 and none leading to study discontinuation. The study was terminated prematurely, as the sponsor made a non-safety-related strategic decision to discontinue rilematovir development prior to full recruitment of Cohort 2.
Conclusions: Data from the combined cohort suggest that rilematovir has a small but favorable antiviral effect of indeterminate clinical relevance compared with placebo, as well as a favorable safety profile. Safe and effective therapeutic options for RSV in infants and young children remain an unmet need.
Clinical trial registration: EudraCT Number: 2016-003642-93; ClinicalTrials.gov Identifier: NCT03656510. First posted date: 4 September, 2018.
© 2024. The Author(s).
Conflict of interest statement
Fernando Ferrero, Chien-Yu Lin, Kleber Luz, Tatyana Stoeva, Agnes Nemeth, Cristina Calvo, and Silvina Natalini have no conflicts of interest that are directly relevant to the content of this article. Johannes Liese received study grants for participation in the CROCuS trial. Manuel Gijón has received a public research grant from a public health institution (Instituto de Salud Carlos III, Madrid, Spain) to develop research tasks, with no conflicts of interests with the present study. Teck-Hock Toh received financial support from the study sponsor for travel to an investigator meeting prior to beginning the study. Sofie Deleu, Sarah Rusch, Leen Vijgen, and Mohamed Gamil are employees of Janssen Research & Development and may own stock or stock options. Bohang Chen is a prior employee of Janssen Research & Development and is a current employee of Bristol Myers Squibb. Beatriz López Sánchez is an employee of Janssen Research & Development under the legal entity Janssen Vaccines & Prevention B.V. Illse Leipoldt is an employee of Janssen-Cilag Pharmaceutical South Africa. Dymphy Huntjens is a prior employee of Janssen Research & Development and is a current employee of Priothera SAS and may own stock or stock options. Tristan Baguet is an employee of Janssen Research & Development. Kristi Bertzos is an employee of Janssen Global Services and holds stock options. Marita Stevens is a prior employee of Janssen Research & Development and may own stock or stock options.
Figures

Similar articles
-
A pilot phase 2a, randomized, double-blind, placebo-controlled study to explore the antiviral activity, clinical outcomes, safety, and tolerability of rilematovir at two dose levels in non-hospitalized adults with respiratory syncytial virus infection.Clin Microbiol Infect. 2023 Oct;29(10):1320-1327. doi: 10.1016/j.cmi.2023.07.004. Epub 2023 Jul 6. Clin Microbiol Infect. 2023. PMID: 37422079 Clinical Trial.
-
Nebulised ALX-0171 for respiratory syncytial virus lower respiratory tract infection in hospitalised children: a double-blind, randomised, placebo-controlled, phase 2b trial.Lancet Respir Med. 2021 Jan;9(1):21-32. doi: 10.1016/S2213-2600(20)30320-9. Epub 2020 Sep 28. Lancet Respir Med. 2021. PMID: 33002427 Clinical Trial.
-
A Randomized, Placebo-Controlled, Respiratory Syncytial Virus Human Challenge Study of the Antiviral Efficacy, Safety, and Pharmacokinetics of RV521, an Inhibitor of the RSV-F Protein.Antimicrob Agents Chemother. 2020 Jan 27;64(2):e01884-19. doi: 10.1128/AAC.01884-19. Print 2020 Jan 27. Antimicrob Agents Chemother. 2020. PMID: 31712214 Free PMC article. Clinical Trial.
-
Immunoglobulin treatment for hospitalised infants and young children with respiratory syncytial virus infection.Cochrane Database Syst Rev. 2019 Aug 26;8(8):CD009417. doi: 10.1002/14651858.CD009417.pub2. Cochrane Database Syst Rev. 2019. Update in: Cochrane Database Syst Rev. 2023 Oct 23;10:CD009417. doi: 10.1002/14651858.CD009417.pub3. PMID: 31446622 Free PMC article. Updated.
-
Systematic Review of the Efficacy and Safety of RSV-Specific Monoclonal Antibodies and Antivirals in Development.Rev Med Virol. 2024 Sep;34(5):e2576. doi: 10.1002/rmv.2576. Rev Med Virol. 2024. PMID: 39209729
Cited by
-
Vaccine and therapeutic agents against the respiratory syncytial virus: resolved and unresolved issue.MedComm (2020). 2024 Nov 21;5(12):e70016. doi: 10.1002/mco2.70016. eCollection 2024 Dec. MedComm (2020). 2024. PMID: 39575302 Free PMC article. Review.
-
Interplay between respiratory viruses and cilia in the airways.Eur Respir Rev. 2025 Mar 19;34(175):240224. doi: 10.1183/16000617.0224-2024. Print 2025 Jan. Eur Respir Rev. 2025. PMID: 40107662 Free PMC article. Review.
References
-
- Center for Disease Control and Prevention. Respiratory syncytial virus infection (RSV). 2022. https://www.cdc.gov/rsv/index.html. Accessed 19 Dec 2022.
-
- Shi T, McAllister DA, O'Brien KL, Simoes EAF, Madhi SA, Gessner BD, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. 2017;390:946–958. doi: 10.1016/S0140-6736(17)30938-8. - DOI - PMC - PubMed
-
- American Academy of Pediatrics. Red book 2018–2021. Report of the Committee of Infectious Diseases. Itasca: American Academy of Pediatrics; 2018.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

