Superior suppression of serum estrogens during neoadjuvant breast cancer treatment with letrozole compared to exemestane
- PMID: 38649619
- PMCID: PMC11182829
- DOI: 10.1007/s10549-024-07313-x
Superior suppression of serum estrogens during neoadjuvant breast cancer treatment with letrozole compared to exemestane
Abstract
Purpose: The aromatase inhibitor letrozole and the aromatase inactivator exemestane are two of the most pivotal cancer drugs used for endocrine treatment of ER-positive breast cancer in all phases of the disease. Although both drugs inhibit CYP19 (aromatase) and have been used for decades, a direct head-to-head, intra-patient-cross-over comparison of their ability to decrease estrogen synthesis in vivo is still lacking.
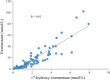
Methods: Postmenopausal breast cancer patients suitable for neoadjuvant endocrine therapy were randomized to receive either letrozole (2.5 mg o.d.) or exemestane (25 mg o.d.) for an initial treatment period, followed by a second treatment period on the alternative drug (intra-patient cross-over study design). Serum levels of estrone (E1), estradiol (E2), letrozole, exemestane, and 17-hydroxyexemestane were quantified simultaneously using a novel, ultrasensitive LC-MS/MS method established in our laboratory.
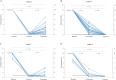
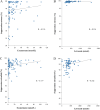
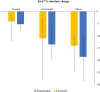
Results: Complete sets of serum samples (baseline and during treatment with letrozole or exemestane) were available from 79 patients, including 40 patients starting with letrozole (cohort 1) and 39 with exemestane (cohort 2). Mean serum estrone and estradiol levels in cohort 1 were 174 pmol/L and 46.4 pmol/L at baseline, respectively. Treatment with letrozole suppressed serum E1 and E2 to a mean value of 0.2 pmol/L and 0.4 pmol/L (P < 0.001). After the cross-over to exemestane, mean serum levels of E1 and E2 increased to 1.4 pmol/L and 0.7 pmol/L, respectively. In cohort 2, baseline mean serum levels of E1 and E2 were 159 and 32.5 pmol/L, respectively. Treatment with exemestane decreased these values to 1.8 pmol/L for E1 and 0.6 pmol/L for E2 (P < 0.001). Following cross-over to letrozole, mean serum levels of E1 and E2 were significantly further reduced to 0.1 pmol/L and 0.4 pmol/L, respectively. Serum drug levels were monitored in all patients throughout the entire treatment and confirmed adherence to the protocol and drug concentrations within the therapeutic range for all patients. Additionally, Ki-67 values decreased significantly during treatment with both aromatase inhibitors, showing a trend toward a stronger suppression in obese women.
Conclusion: To the best of our knowledge, we present here for the first time a comprehensive and direct head-to-head, intra-patient-cross-over comparison of the aromatase inhibitor letrozole and the aromatase inactivator exemestane concerning their ability to suppress serum estrogen levels in vivo. All in all, our results clearly demonstrate that letrozole therapy results in a more profound suppression of serum E1 and E2 levels compared to exemestane.
Keywords: Aromatase; Breast cancer; Estrogens; Exemestane; Letrozole; Neoadjuvant.
© 2024. The Author(s).
Conflict of interest statement
The authors have not disclosed any competing interests.
Figures



References
-
- Miller WR. Aromatase inhibitors and breast cancer. Minerva Endocrinol. 2006;31(1):27–46. - PubMed
-
- Geisler J, King N, Dowsett M, Ottestad L, Lundgren S, Walton P, Kormeset PO, Lonning PE. Influence of anastrozole (Arimidex), a selective, non-steroidal aromatase inhibitor, on in vivo aromatisation and plasma oestrogen levels in postmenopausal women with breast cancer. Br J Cancer. 1996;74(8):1286–1291. doi: 10.1038/bjc.1996.531. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

