Merkel Cell Carcinoma: Integrating Epidemiology, Immunology, and Therapeutic Updates
- PMID: 38649621
- PMCID: PMC11193695
- DOI: 10.1007/s40257-024-00858-z
Merkel Cell Carcinoma: Integrating Epidemiology, Immunology, and Therapeutic Updates
Abstract
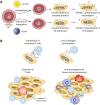
Merkel cell carcinoma (MCC) is a rare skin cancer characterized by neuroendocrine differentiation. Its carcinogenesis is based either on the integration of the Merkel cell polyomavirus or on ultraviolet (UV) mutagenesis, both of which lead to high immunogenicity either through the expression of viral proteins or neoantigens. Despite this immunogenicity resulting from viral or UV-associated carcinogenesis, it exhibits highly aggressive behavior. However, owing to the rarity of MCC and the lack of epidemiologic registries with detailed clinical data, there is some uncertainty regarding the spontaneous course of the disease. Historically, advanced MCC patients were treated with conventional cytotoxic chemotherapy yielding a median response duration of only 3 months. Starting in 2017, four programmed cell death protein 1 (PD-1)/programmed cell death-ligand 1 (PD-L1) immune checkpoint inhibitors-avelumab, pembrolizumab, nivolumab (utilized in both neoadjuvant and adjuvant settings), and retifanlimab-have demonstrated efficacy in treating patients with disseminated MCC on the basis of prospective clinical trials. However, generating clinical evidence for rare cancers, such as MCC, is challenging owing to difficulties in conducting large-scale trials, resulting in small sample sizes and therefore lacking statistical power. Thus, to comprehensively understand the available clinical evidence on various immunotherapy approaches for MCC, we also delve into the epidemiology and immune biology of this cancer. Nevertheless, while randomized studies directly comparing immune checkpoint inhibitors and chemotherapy in MCC are lacking, immunotherapy shows response rates comparable to those previously reported with chemotherapy but with more enduring responses. Notably, adjuvant nivolumab has proven superiority to the standard-of-care therapy (observation) in the adjuvant setting.
© 2024. The Author(s).
Conflict of interest statement
JCB is receiving speaker’s bureau honoraria from Amgen, Pfizer, and Sanofi and is a paid consultant/advisory board member/data and safety monitoring board (DSMB) member for Almirall, Boehringer Ingelheim, ICON, Merck, Pfizer, and Sanofi. His group receives research grants from Merck, HTG, IQVIA, and Alcedis. SU declares research support from Bristol Myers Squibb and Merck; speaker and advisory board honoraria from Bristol Myers Squibb, Merck Sharp & Dohme, Merck Serono, and Novartis; and meeting and travel support from Almirall, Bristol-Myers Squibb, IGEA Clinical Biophysics, Merck Sharp & Dohme, Novartis, Pierre Fabre, and Sun Pharma. AS and DS declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

