Development of a Weight-Band Dosing Approach for Vosoritide in Children with Achondroplasia Using a Population Pharmacokinetic Model
- PMID: 38649657
- PMCID: PMC11106139
- DOI: 10.1007/s40262-024-01371-6
Development of a Weight-Band Dosing Approach for Vosoritide in Children with Achondroplasia Using a Population Pharmacokinetic Model
Abstract
Background and objective: Vosoritide is a recently approved therapy for achondroplasia, the most common form of disproportionate short stature, that has been shown to be well tolerated and effective in increasing linear growth. This study aimed to develop a population pharmacokinetic (PPK) model to characterize pharmacokinetics (PK) of vosoritide and establish a weight-band dosing regimen.
Methods: A PPK model was developed using data from five clinical trials in children with achondroplasia (aged 0.95-15 years) who received daily per-kg doses of vosoritide. The model was used to simulate expected exposures in children with a refined weight-band dosing regimen. Simulated exposure was compared with the observed exposure from the pivotal clinical trial to evaluate appropriateness of the weight-band dosing regimen.
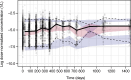
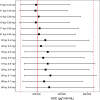
Results: A one-compartment model with a change-point first-order absorption and first-order elimination accurately described PK of vosoritide in children with achondroplasia. Body weight was found to be a predictor of vosoritide's clearance and volume of distribution. Additionally, it was observed that dosing solution concentration and duration of treatment influenced bioavailability. The weight-band dosing regimen resulted in simulated exposures that were within the range demonstrated to be well tolerated and effective in the pivotal clinical trial and showed improved consistency in drug exposure across the achondroplasia population.
Conclusions: The weight-band dosing regimen reduced the number of recommended dose levels by body weight and is expected to simplify dosing for children with achondroplasia and their caregivers.
Clinical trial registration: NCT02055157, NCT02724228, NCT03197766, NCT03424018, and NCT03583697.
© 2024. The Author(s).
Conflict of interest statement
Y.Q., K.L., E.F., A.C., J.D., and J.H.: employees and shareholders of BioMarin Pharmaceutical Inc. M.L.C.: former employee and stockholder of BioMarin Pharmaceutical Inc. D.R.M.: paid consultant for BioMarin Pharmaceutical Inc. R.S. and K.O.: honoraria from BioMarin Pharmaceutical Inc. M.I.: honoraria for consultancy services relating to the subject of this manuscript. C.A.B. and K.M.: no conflicts of interest. J.H.-.F.: institution received funding from BioMarin Pharmaceutical Inc. to execute the study presented in this manuscript. Funds were also received from Pfizer and QED for other achondroplasia-related studies. J.H.-F.: consultant for BioMarin Pharmaceutical Inc., Pfizer, QED, and Sanofi, related to achondroplasia. W.R.W.: local principal investigator for the vosoritide studies at Emory University, funded by a clinical trial contract from BioMarin Pharmaceutical Inc. to Emory University. W.R.W. received honoraria for advisory committee meetings from BioMarin Pharmaceutical Inc. BioMarin Pharmaceutical Inc. paid for writing assistance for the manuscript. M.B.B.: employed by the Nemours Foundation, which has received institutional support for research from Ascendis Pharma, BioMarin Pharmaceutical Inc., QED, and Therachon/Pfizer. His institution has received consulting fees from BioMarin Pharmaceutical Inc. and Tyra Biosciences. M.B.B. has received consulting fees from Ascendis Pharma, BioMarin Pharmaceutical Inc., QED, and Therachon/Pfizer. He has received compensation for speaking on behalf of the Alexion speaker’s bureau and from Novo Nordisk and Tyra Biosciences. In the future, he will be employed by Tyra Biosciences.
Figures


References
-
- Vajo Z, Francomano CA, Wilkin DJ. The molecular and genetic basis of fibroblast growth factor receptor 3 disorders: the achondroplasia family of skeletal dysplasias, Muenke craniosynostosis, and Crouzon syndrome with acanthosis nigricans. Endocr Rev. 2000;21:23–39. doi: 10.1210/edrv.21.1.0387. - DOI - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

