Evaluation of angiotensin converting enzyme 2 (ACE2), angiotensin II (Ang II), miR-141-3p, and miR-421 levels in SARS-CoV-2 patients: a case-control study
- PMID: 38649818
- PMCID: PMC11036566
- DOI: 10.1186/s12879-024-09310-3
Evaluation of angiotensin converting enzyme 2 (ACE2), angiotensin II (Ang II), miR-141-3p, and miR-421 levels in SARS-CoV-2 patients: a case-control study
Abstract
Background: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a highly contagious virus that uses angiotensin converting enzyme 2 (ACE2), a pivotal member of the renin-angiotensin system (RAS), as its cell-entry receptor. Another member of the RAS, angiotensin II (Ang II), is the major biologically active component in this system. There is growing evidence suggesting that serum miRNAs could serve as prognostic biomarkers for SARS-CoV-2 infection and regulate ACE2 expression. Therefore, the aim of this study is to evaluate the changes in the serum levels of sACE2 and Ang II, as well as the expression level of miR-141-3p and miR-421 in SARS-CoV-2 positive and negative subjects.
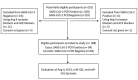
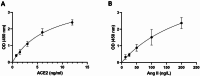
Methods: In the present study, the serum levels of sACE2 and Ang II were measured in 94 SARS-CoV-2 positive patients and 94 SARS-CoV-2 negative subjects with some symptoms similar to those of SARS-CoV-2 positive patients using the ELISA method. In addition, the expression level of miR-141-3p and miR-421 as ACE2 regulators and biomarkers was evaluated using quantitative real-time PCR (qRT-PCR) method.
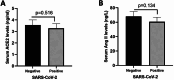
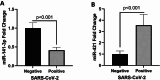
Results: The mean serum sACE2 concentration in the SARS-CoV-2-positive group was 3.268 ± 0.410 ng/ml, whereas in the SARS-CoV-2 negative group, it was 3.564 ± 0.437 ng/ml. Additionally, the mean serum Ang II level in the SARS-CoV-2 positive and negative groups were 60.67 ± 6.192 ng/L and 67.97 ± 6.837 ng/L, respectively. However, there was no significant difference in the serum levels of sACE2 (P value: 0.516) and Ang II (P value: 0.134) between the SARS-CoV-2 positive and negative groups. Meanwhile, our findings indicated that the expression levels of miR-141-3p and miR-421 in SARS-CoV-2 positive group were significantly lower and higher than SARS-CoV-2 negative group, respectively (P value < 0.001).
Conclusions: Taken together, the results of this study showed that the serum levels of sACE2 and Ang II in SARS-CoV-2 positive and negative subjects were not significantly different, but the expression levels of miR-141-3p and miR-421 were altered in SARS-CoV-2 positive patients which need more investigation to be used as biomarkers for COVID-19 diagnosis.
Keywords: Angiotensin II; Angiotensin converting enzyme 2; COVID-19; SARS-CoV-2; miR-141-3p; miR-421.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Expression of ACE2, Soluble ACE2, Angiotensin I, Angiotensin II and Angiotensin-(1-7) Is Modulated in COVID-19 Patients.Front Immunol. 2021 Jun 14;12:625732. doi: 10.3389/fimmu.2021.625732. eCollection 2021. Front Immunol. 2021. PMID: 34194422 Free PMC article.
-
Circulating Soluble ACE2 and Upstream microRNA Expressions in Serum of Type 2 Diabetes Mellitus Patients.Int J Mol Sci. 2021 May 17;22(10):5263. doi: 10.3390/ijms22105263. Int J Mol Sci. 2021. PMID: 34067683 Free PMC article.
-
Potential detrimental role of soluble ACE2 in severe COVID-19 comorbid patients.Rev Med Virol. 2021 Sep;31(5):1-12. doi: 10.1002/rmv.2213. Epub 2021 Jan 10. Rev Med Virol. 2021. PMID: 33426683 Free PMC article. Review.
-
Soluble angiotensin-converting enzyme 2 is transiently elevated in COVID-19 and correlates with specific inflammatory and endothelial markers.J Med Virol. 2021 Oct;93(10):5908-5916. doi: 10.1002/jmv.27144. Epub 2021 Jul 6. J Med Virol. 2021. PMID: 34138483 Free PMC article.
-
ACE2 Shedding and the Role in COVID-19.Front Cell Infect Microbiol. 2022 Jan 14;11:789180. doi: 10.3389/fcimb.2021.789180. eCollection 2021. Front Cell Infect Microbiol. 2022. PMID: 35096642 Free PMC article. Review.
Cited by
-
Evaluating the Role of the Renin-angiotensin System in COVID-19: Implications for ACE Inhibitor and ARB Use During SARS-CoV-2 Infection.J Cell Immunol. 2024;6(6):255-265. doi: 10.33696/immunology.6.213. J Cell Immunol. 2024. PMID: 40417281 Free PMC article.
-
Synthesis, molecular docking, and in vitro activity of a novel angiotensin-converting enzyme 2 inhibitor, LMS1007: a potential molecule in Covid-19 and cancer treatments.RSC Adv. 2025 May 8;15(19):15138-15154. doi: 10.1039/d5ra01134e. eCollection 2025 May 6. RSC Adv. 2025. PMID: 40343305 Free PMC article.
References
-
- Basavaraju SV, Patton ME, Grimm K, Rasheed MAU, Lester S, Mills L, et al. Serologic Testing of US blood donations to identify severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)–Reactive antibodies: December 2019–January 2020. Clin Infect Dis. 2020;72(12):e1004–9. doi: 10.1093/cid/ciaa1785. - DOI - PMC - PubMed
-
- Merad M, Blish CA, Sallusto F, Iwasaki A. The immunology and immunopathology of COVID-19. Science (New York, NY). 2022;375(6585):1122-7. - PubMed
-
- Shatizadeh Malekshahi S, Yavarian J, Shafiei-Jandaghi NZ. Usage of peptidases by SARS-CoV-2 and several human coronaviruses as receptors: a mysterious story. Biotechnol Appl Chem. 2022;69(1):124–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

