Endoscopic manifestation of intestinal transplant-associated microangiopathy after stem cell transplantation
- PMID: 38649868
- PMCID: PMC11034059
- DOI: 10.1186/s12876-024-03221-y
Endoscopic manifestation of intestinal transplant-associated microangiopathy after stem cell transplantation
Abstract
Background: Endoscopic features of intestinal transplant-associated microangiopathy (iTAM) have not been comprehensively investigated. This study aimed to examine the endoscopic characteristics of patients diagnosed with iTAM.
Methods: This retrospective analysis included 14 patients pathologically diagnosed with iTAM after stem cell transplantation for hematolymphoid neoplasms (n = 13) or thalassemia (n = 1). The sex, age at diagnosis, endoscopic features, and prognosis of each patient were assessed. Serological markers for diagnosing transplant-associated thrombotic microangiopathy were also evaluated.
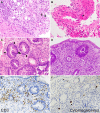
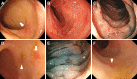
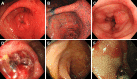
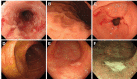
Results: The mean age at the time of iTAM diagnosis was 40.2 years. Patients diagnosed based on the pathognomonic pathological changes of iTAM presented with diverse symptoms at the times of endoscopic examinations, including diarrhea (n = 10), abdominal pain (n = 5), nausea (n = 4), appetite loss (n = 2), bloody stools (n = 2), abdominal discomfort (n = 1), and vomiting (n = 1). At the final follow-up, six patients survived, while eight patients succumbed, with a median time of 100.5 days (range: 52-247) post-diagnosis. Endoscopic manifestations included erythematous mucosa (n = 14), erosions (n = 13), ulcers (n = 9), mucosal edema (n = 9), granular mucosa (n = 9), and villous atrophy (n = 4). Erosions and/or ulcers were primarily observed in the colon (10/14, 71%), followed by the ileum (9/13, 69%), stomach (4/10, 40%), cecum (5/14, 36%), duodenum (3/10, 30%), rectum (4/14, 29%), and esophagus (1/10, 10%). Cytomegalovirus infection (n = 4) and graft-versus-host disease (n = 2) coexisted within the gastrointestinal tract. Patients had de novo prolonged or progressive thrombocytopenia (6/14, 43%), decreased hemoglobin concentration (4/14, 29%), reduced serum haptoglobin level (3/14, 21%), and a sudden and persistent increase in lactate dehydrogenase level (2/14, 14%). Peripheral blood samples from 12 patients were evaluated for schistocytes, with none exceeding 4%.
Conclusions: This study provides a comprehensive exploration of the endoscopic characteristics of iTAM. Notably, all patients exhibited erythematous mucosa throughout the gastrointestinal tract, accompanied by prevalent manifestations, such as erosions (93%), ulcers (64%), mucosal edema (64%), granular mucosa (64%), and villous atrophy (29%). Because of the low positivity for serological markers of transplant-associated thrombotic microangiopathy in patients with iTAM, endoscopic evaluation and biopsy of these lesions are crucial, even in the absence of these serological features.
Keywords: Colonoscopy; Esophagogastroduodenoscopy; Graft-versus-host disease; Hematopoietic stem cell transplantation; Intestinal transplant-associated microangiopathy; iTAM.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
A Complete Histologic Approach to Gastrointestinal Biopsy From Hematopoietic Stem Cell Transplant Patients With Evidence of Transplant-Associated Gastrointestinal Thrombotic Microangiopathy.Arch Pathol Lab Med. 2017 Nov;141(11):1558-1566. doi: 10.5858/arpa.2016-0599-RA. Epub 2017 Aug 10. Arch Pathol Lab Med. 2017. PMID: 28795840
-
Histologic Features of Intestinal Thrombotic Microangiopathy in Pediatric and Young Adult Patients after Hematopoietic Stem Cell Transplantation.Biol Blood Marrow Transplant. 2015 Nov;21(11):1994-2001. doi: 10.1016/j.bbmt.2015.06.016. Epub 2015 Jul 4. Biol Blood Marrow Transplant. 2015. PMID: 26150023 Free PMC article.
-
Intestinal thrombotic microangiopathy: a distinct entity in the spectrum of graft-versus-host disease.Int J Hematol. 2019 Nov;110(5):529-532. doi: 10.1007/s12185-019-02750-7. Epub 2019 Oct 4. Int J Hematol. 2019. PMID: 31586304
-
Diagnosis of intestinal graft-versus-host disease and thrombotic microangiopathy after allogeneic stem cell transplantation.Tohoku J Exp Med. 2012 May;227(1):31-7. doi: 10.1620/tjem.227.31. Tohoku J Exp Med. 2012. PMID: 22531159
-
Hematopoietic Stem Cell Transplant-Associated Thrombotic Microangiopathy.Clin Appl Thromb Hemost. 2016 Jan;22(1):12-20. doi: 10.1177/1076029615598221. Epub 2015 Aug 2. Clin Appl Thromb Hemost. 2016. PMID: 26239316 Review.
Cited by
-
Small Intestinal Complications of Intestinal Transplant-Associated Microangiopathy Post-stem Cell Transplantation.Cureus. 2024 Jul 8;16(7):e64111. doi: 10.7759/cureus.64111. eCollection 2024 Jul. Cureus. 2024. PMID: 39114192 Free PMC article.
References
-
- Warren M, Jodele S, Dandoy C, Myers KC, Wallace G, Nelson A, et al. A complete histologic Approach to Gastrointestinal Biopsy from hematopoietic stem cell transplant patients with evidence of Transplant-Associated gastrointestinal thrombotic microangiopathy. Arch Pathol Lab Med. 2017;141:1558–66. doi: 10.5858/arpa.2016-0599-RA. - DOI - PubMed
-
- El-Bietar J, Warren M, Dandoy C, Myers KC, Lane A, Wallace G, et al. Histologic features of intestinal thrombotic Microangiopathy in Pediatric and Young Adult patients after hematopoietic stem cell transplantation. Biol Blood Marrow Transpl. 2015;21:1994–2001. doi: 10.1016/j.bbmt.2015.06.016. - DOI - PMC - PubMed
-
- Chapin J, Shore T, Forsberg P, Desman G, Van Besien K, Laurence J. Hematopoietic transplant-associated thrombotic microangiopathy: case report and review of diagnosis and treatments. Clin Adv Hematol Oncol. 2014;12:565–73. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

