CircPRKD3/miR-6783-3p responds to mechanical force to facilitate the osteogenesis of stretched periodontal ligament stem cells
- PMID: 38649946
- PMCID: PMC11036753
- DOI: 10.1186/s13018-024-04727-7
CircPRKD3/miR-6783-3p responds to mechanical force to facilitate the osteogenesis of stretched periodontal ligament stem cells
Abstract
Background: The mechanotransduction mechanisms by which cells regulate tissue remodeling are not fully deciphered. Circular RNAs (circRNAs) are crucial to various physiological processes, including cell cycle, differentiation, and polarization. However, the effects of mechanical force on circRNAs and the role of circRNAs in the mechanobiology of differentiation and remodeling in stretched periodontal ligament stem cells (PDLSCs) remain unclear. This article aims to explore the osteogenic function of mechanically sensitive circular RNA protein kinase D3 (circPRKD3) and elucidate its underlying mechanotransduction mechanism.
Materials and methods: PDLSCs were elongated with 8% stretch at 0.5 Hz for 24 h using the Flexcell® FX-6000™ Tension System. CircPRKD3 was knockdown or overexpressed with lentiviral constructs or plasmids. The downstream molecules of circPRKD3 were predicted by bioinformatics analysis. The osteogenic effect of related molecules was evaluated by quantitative real-time PCR (qRT-PCR) and western blot.
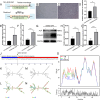
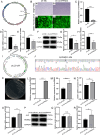
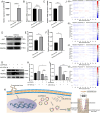
Results: Mechanical force enhanced the osteogenesis of PDLSCs and increased the expression of circPRKD3. Knockdown of circPRKD3 hindered PDLSCs from osteogenesis under mechanical force, while overexpression of circPRKD3 promoted the early osteogenesis process of PDLSCs. With bioinformatics analysis and multiple software predictions, we identified hsa-miR-6783-3p could act as the sponge of circPRKD3 to indirectly regulate osteogenic differentiation of mechanically stimulated PDLSCs.
Conclusions: Our results first suggested that both circPRKD3 and hsa-miR-6783-3p could enhance osteogenesis of stretched PDLSCs. Furthermore, hsa-miR-6783-3p could sponge circPRKD3 to indirectly regulate RUNX2 during the periodontal tissue remodeling process in orthodontic treatment.
Keywords: CircPRKD3; Mechanical force; PDLSCs; ceRNAs; miR-6783-3p.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Liu M, Zhang H, Li Y, Wang S. Noncoding RNAs interplay in Ovarian Cancer Therapy and Drug Resistance. Cancer biotherapy & radiopharmaceuticals; 2022. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

