Refined protocol for newly onset identification in non-obese diabetic mice: an animal-friendly, cost-effective, and efficient alternative
- PMID: 38649958
- PMCID: PMC11034171
- DOI: 10.1186/s42826-024-00202-w
Refined protocol for newly onset identification in non-obese diabetic mice: an animal-friendly, cost-effective, and efficient alternative
Abstract
Background: Therapeutic interventions for diabetes are most effective when administered in the newly onset phase, yet determining the exact onset moment can be elusive in practice. Spontaneous autoimmune diabetes among NOD mice appears randomly between 12 and 32 weeks of age with an incidence range from 60 to 90%. Furthermore, the disease often progresses rapidly to severe diabetes within days, resulting in a very short window of newly onset phase, that poses significant challenge in early diagnosis. Conventionally, extensive blood glucose (BG) testing is typically required on large cohorts throughout several months to conduct prospective survey. We incorporated ultrasensitive urine glucose (UG) testing into an ordinary BG survey process, initially aiming to elucidate the lag period required for excessive glucose leaking from blood to urine during diabetes progression in the mouse model.
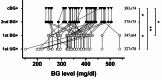
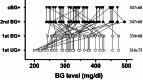
Results: The observations unexpectedly revealed that small amounts of glucose detected in the urine often coincide with, sometimes even a couple days prior than elevated BG is diagnosed. Accordingly, we conducted the UG-based survey protocol in another cohort that was validated to accurately identified every individual near onset, who could then be confirmed by following few BG tests to fulfill the consecutive BG + criteria. This approach required fewer than 95 BG tests, compared to over 700 tests with traditional BG survey, to diagnose all the 37-38 diabetic mice out of total 60. The average BG level at diagnosis was slightly below 350 mg/dl, lower than the approximately 400 mg/dl observed with conventional BG monitoring.
Conclusions: We demonstrated a near perfect correlation between BG + and ultrasensitive UG + results in prospective survey with no lag period detected under twice weekly of testing frequency. This led to the refined protocol based on surveying with noninvasive UG testing, allowing for the early identification of newly onset diabetic mice with only a few BG tests required per mouse. This protocol significantly reduces the need for extensive blood sampling, lancet usage, labor, and animal distress, aligning with the 3Rs principle. It presents a convenient, accurate, and animal-friendly alternative for early diabetes diagnosis, facilitating research on diagnosis, pathogenesis, prevention, and treatment.
Keywords: Autoimmune diabetes; Blood glucose; Early onset diagnosis; Glycemia; Glycosuria; NOD mice; Spontaneous diabetes; Ultrasensitive glucose test; Urine glucose.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests.
Figures




References
LinkOut - more resources
Full Text Sources

