Promoting osteogenesis and bone regeneration employing icariin-loaded nanoplatforms
- PMID: 38649969
- PMCID: PMC11036660
- DOI: 10.1186/s13036-024-00425-4
Promoting osteogenesis and bone regeneration employing icariin-loaded nanoplatforms
Abstract
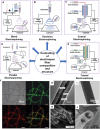
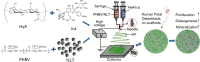
There is an increasing demand for innovative strategies that effectively promote osteogenesis and enhance bone regeneration. The critical process of bone regeneration involves the transformation of mesenchymal stromal cells into osteoblasts and the subsequent mineralization of the extracellular matrix, making up the complex mechanism of osteogenesis. Icariin's diverse pharmacological properties, such as anti-inflammatory, anti-oxidant, and osteogenic effects, have attracted considerable attention in biomedical research. Icariin, known for its ability to stimulate bone formation, has been found to encourage the transformation of mesenchymal stromal cells into osteoblasts and improve the subsequent process of mineralization. Several studies have demonstrated the osteogenic effects of icariin, which can be attributed to its hormone-like function. It has been found to induce the expression of BMP-2 and BMP-4 mRNAs in osteoblasts and significantly upregulate Osx at low doses. Additionally, icariin promotes bone formation by stimulating the expression of pre-osteoblastic genes like Osx, RUNX2, and collagen type I. However, icariin needs to be effectively delivered to bone to perform such promising functions.Encapsulating icariin within nanoplatforms holds significant promise for promoting osteogenesis and bone regeneration through a range of intricate biological effects. When encapsulated in nanofibers or nanoparticles, icariin exerts its effects directly at the cellular level. Recalling that inflammation is a critical factor influencing bone regeneration, icariin's anti-inflammatory effects can be harnessed and amplified when encapsulated in nanoplatforms. Also, while cell adhesion and cell migration are pivotal stages of tissue regeneration, icariin-loaded nanoplatforms contribute to these processes by providing a supportive matrix for cellular attachment and movement. This review comprehensively discusses icariin-loaded nanoplatforms used for bone regeneration and osteogenesis, further presenting where the field needs to go before icariin can be used clinically.
Keywords: Bone regeneration; Icariin; Nanofibers; Nanoparticles; Nanoplatforms; Osteogenesis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







Similar articles
-
Icariin promotes bone marrow mesenchymal stem cells osteogenic differentiation via the mTOR/autophagy pathway to improve ketogenic diet-associated osteoporosis.J Orthop Surg Res. 2024 Feb 7;19(1):127. doi: 10.1186/s13018-024-04529-x. J Orthop Surg Res. 2024. PMID: 38326818 Free PMC article.
-
Icariin-loaded porous scaffolds for bone regeneration through the regulation of the coupling process of osteogenesis and osteoclastic activity.Int J Nanomedicine. 2019 Aug 1;14:6019-6033. doi: 10.2147/IJN.S203859. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31534334 Free PMC article.
-
Icariin promotes osteogenic differentiation of rat bone marrow stromal cells by activating the ERα-Wnt/β-catenin signaling pathway.Biomed Pharmacother. 2016 Dec;84:931-939. doi: 10.1016/j.biopha.2016.09.107. Epub 2016 Oct 17. Biomed Pharmacother. 2016. PMID: 27764755
-
Periosteum and development of the tissue-engineered periosteum for guided bone regeneration.J Orthop Translat. 2022 Feb 16;33:41-54. doi: 10.1016/j.jot.2022.01.002. eCollection 2022 Mar. J Orthop Translat. 2022. PMID: 35228996 Free PMC article. Review.
-
Effects of nanofibers on mesenchymal stem cells: environmental factors affecting cell adhesion and osteogenic differentiation and their mechanisms.J Zhejiang Univ Sci B. 2020 Nov.;21(11):871-884. doi: 10.1631/jzus.B2000355. J Zhejiang Univ Sci B. 2020. PMID: 33150771 Free PMC article. Review.
Cited by
-
The Buccal Fat Pad: A Unique Human Anatomical Structure and Rich and Easily Accessible Source of Mesenchymal Stem Cells for Tissue Repair.Bioengineering (Basel). 2024 Sep 27;11(10):968. doi: 10.3390/bioengineering11100968. Bioengineering (Basel). 2024. PMID: 39451344 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

