Dengue virus pathogenesis and host molecular machineries
- PMID: 38649998
- PMCID: PMC11036733
- DOI: 10.1186/s12929-024-01030-9
Dengue virus pathogenesis and host molecular machineries
Abstract
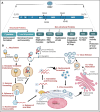
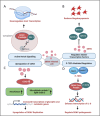
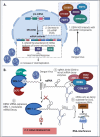
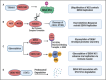
Dengue viruses (DENV) are positive-stranded RNA viruses belonging to the Flaviviridae family. DENV is the causative agent of dengue, the most rapidly spreading viral disease transmitted by mosquitoes. Each year, millions of people contract the virus through bites from infected female mosquitoes of the Aedes species. In the majority of individuals, the infection is asymptomatic, and the immune system successfully manages to control virus replication within a few days. Symptomatic individuals may present with a mild fever (Dengue fever or DF) that may or may not progress to a more critical disease termed Dengue hemorrhagic fever (DHF) or the fatal Dengue shock syndrome (DSS). In the absence of a universally accepted prophylactic vaccine or therapeutic drug, treatment is mostly restricted to supportive measures. Similar to many other viruses that induce acute illness, DENV has developed several ways to modulate host metabolism to create an environment conducive to genome replication and the dissemination of viral progeny. To search for new therapeutic options, understanding the underlying host-virus regulatory system involved in various biological processes of the viral life cycle is essential. This review aims to summarize the complex interaction between DENV and the host cellular machinery, comprising regulatory mechanisms at various molecular levels such as epigenetic modulation of the host genome, transcription of host genes, translation of viral and host mRNAs, post-transcriptional regulation of the host transcriptome, post-translational regulation of viral proteins, and pathways involved in protein degradation.
Keywords: Dengue virus; Epigenomic regulation; Post-transcriptional modifications; Post-translational modifications; Stress granule formation; Transcription regulation; Translation regulation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Libraty DH, Young PR, Pickering D, Endy TP, Kalayanarooj S, Green S, et al. High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J Infect Dis. 2002;186(8):1165–1168. doi: 10.1086/343813. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

