Predictive significance of FGFR4 p.G388R polymorphism in metastatic colorectal cancer patients receiving trifluridine/tipiracil (TAS-102) treatment
- PMID: 38650006
- PMCID: PMC11036552
- DOI: 10.1186/s12967-024-05184-w
Predictive significance of FGFR4 p.G388R polymorphism in metastatic colorectal cancer patients receiving trifluridine/tipiracil (TAS-102) treatment
Abstract
Background: TAS-102 (Lonsurf®) is an oral fluoropyrimidine consisting of a combination of trifluridine (a thymidine analog) and tipiracil (a thymidine phosphorylation inhibitor). The drug is effective in metastatic colorectal cancer (mCRC) patients refractory to fluorouracil, irinotecan and oxaliplatin. This study is a real-world analysis, investigating the interplay of genotype/phenotype in relation to TAS-102 sensitivity.
Methods: Forty-seven consecutive mCRC patients were treated with TAS-102 at the National Cancer Institute of Naples from March 2019 to March 2021, at a dosage of 35 mg/m2, twice a day, in cycles of 28 days (from day 1 to 5 and from day 8 to 12). Clinical-pathological parameters were described. Activity was evaluated with RECIST criteria (v1.1) and toxicity with NCI-CTC (v5.0). Survival was depicted through the Kaplan-Meyer curves. Genetic features of patients were evaluated with Next Generation Sequencing (NGS) through the Illumina NovaSeq 6000 platform and TruSigt™Oncology 500 kit.
Results: Median age of patients was 65 years (range: 46-77). Forty-one patients had 2 or more metastatic sites and 38 patients underwent to more than 2 previous lines of therapies. ECOG (Eastern Cooperative Oncology Group) Performance Status (PS) was 2 in 19 patients. The median number of TAS-102 cycles was 4 (range: 2-12). The most frequent toxic event was neutropenia (G3/G4 in 16 patients). There were no severe (> 3) non-haematological toxicities or treatment-related deaths. Twenty-six patients experienced progressive disease (PD), 21 stable disease (SD). Three patients with long-lasting disease control (DC: complete, partial responses or stable disease) shared an FGFR4 (p.Gly388Arg) mutation. Patients experiencing DC had more frequently a low tumour growth rate (P = 0.0306) and an FGFR4 p.G388R variant (P < 0.0001). The FGFR4 Arg388 genotype was associated with better survival (median: 6.4 months) compared to the Gly388 genotype (median: 4 months); the HR was 0.25 (95% CI 0.12- 0.51; P = 0.0001 at Log-Rank test).
Conclusions: This phenotype/genotype investigation suggests that the FGFR4 p.G388R variant may serve as a new marker for identifying patients who are responsive to TAS-102. A mechanistic hypothesis is proposed to interpret these findings.
Keywords: Colorectal cancer; FGFR4; NGS; TAS-102.
© 2024. The Author(s).
Conflict of interest statement
Roberto Sirica, Giovanni Savarese, Monica Ianniello, and Marika Casillo are employed by AMES, Centro Polidiagnostico Strumentale srl, 80013 Naples, Italy. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential competing interest.
Figures
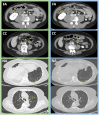

References
-
- Patel MR, Bendell JC, Mayer RJ, et al. A phase I dose-escalation study of TAS-102 in patients (pts) with refractory metastatic colorectal cancer (mCRC) J Clin Oncol. 2012;30:3631–3631. doi: 10.1200/jco.2012.30.15_suppl.3631. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

