Targeting NLRP3 signaling reduces myocarditis-induced arrhythmogenesis and cardiac remodeling
- PMID: 38650023
- PMCID: PMC11034044
- DOI: 10.1186/s12929-024-01032-7
Targeting NLRP3 signaling reduces myocarditis-induced arrhythmogenesis and cardiac remodeling
Abstract
Background: Myocarditis substantially increases the risk of ventricular arrhythmia. Approximately 30% of all ventricular arrhythmia cases in patients with myocarditis originate from the right ventricular outflow tract (RVOT). However, the role of NLRP3 signaling in RVOT arrhythmogenesis remains unclear.
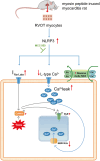
Methods: Rats with myosin peptide-induced myocarditis (experimental group) were treated with an NLRP3 inhibitor (MCC950; 10 mg/kg, daily for 14 days) or left untreated. Then, they were subjected to electrocardiography and echocardiography. Ventricular tissue samples were collected from each rat's RVOT, right ventricular apex (RVA), and left ventricle (LV) and examined through conventional microelectrode and histopathologic analyses. In addition, whole-cell patch-clamp recording, confocal fluorescence microscopy, and Western blotting were performed to evaluate ionic currents, intracellular Ca2+ transients, and Ca2+-modulated protein expression in individual myocytes isolated from the RVOTs.
Results: The LV ejection fraction was lower and premature ventricular contraction frequency was higher in the experimental group than in the control group (rats not exposed to myosin peptide). Myocarditis increased the infiltration of inflammatory cells into cardiac tissue and upregulated the expression of NLRP3; these observations were more prominent in the RVOT and RVA than in the LV. Furthermore, experimental rats treated with MCC950 (treatment group) improved their LV ejection fraction and reduced the frequency of premature ventricular contraction. Histopathological analysis revealed higher incidence of abnormal automaticity and pacing-induced ventricular tachycardia in the RVOTs of the experimental group than in those of the control and treatment groups. However, the incidences of these conditions in the RVA and LV were similar across the groups. The RVOT myocytes of the experimental group exhibited lower Ca2+ levels in the sarcoplasmic reticulum, smaller intracellular Ca2+ transients, lower L-type Ca2+ currents, larger late Na+ currents, larger Na+-Ca2+ exchanger currents, higher reactive oxygen species levels, and higher Ca2+/calmodulin-dependent protein kinase II levels than did those of the control and treatment groups.
Conclusion: Myocarditis may increase the rate of RVOT arrhythmogenesis, possibly through electrical and structural remodeling. These changes may be mitigated by inhibiting NLRP3 signaling.
Keywords: Myocarditis; NLRP3; Right ventricular outflow tract; Ventricular tachycardia.
© 2024. The Author(s).
Conflict of interest statement
All authors declare no competing interest.
Figures






Similar articles
-
Distinctive electrophysiological characteristics of right ventricular out-flow tract cardiomyocytes.J Cell Mol Med. 2014 Aug;18(8):1540-8. doi: 10.1111/jcmm.12329. Epub 2014 Jun 9. J Cell Mol Med. 2014. PMID: 24913286 Free PMC article.
-
Nicotine Exacerbates Arrhythmogenesis in Rabbit Right Ventricular Outflow Tract Triggered by Chronic Obstructive Pulmonary Disease.J Cell Mol Med. 2025 Jun;29(12):e70664. doi: 10.1111/jcmm.70664. J Cell Mol Med. 2025. PMID: 40533933 Free PMC article.
-
Adenosine monophosphate-regulated protein kinase inhibition modulates electrophysiological characteristics and calcium homeostasis of rabbit right ventricular outflow tract.Fundam Clin Pharmacol. 2024 Apr;38(2):262-275. doi: 10.1111/fcp.12953. Epub 2023 Sep 4. Fundam Clin Pharmacol. 2024. PMID: 37664898
-
The covalent NLRP3-inflammasome inhibitor Oridonin relieves myocardial infarction induced myocardial fibrosis and cardiac remodeling in mice.Int Immunopharmacol. 2021 Jan;90:107133. doi: 10.1016/j.intimp.2020.107133. Epub 2020 Nov 7. Int Immunopharmacol. 2021. PMID: 33168408
-
Electrical and Structural Insights into Right Ventricular Outflow Tract Arrhythmogenesis.Int J Mol Sci. 2023 Jul 22;24(14):11795. doi: 10.3390/ijms241411795. Int J Mol Sci. 2023. PMID: 37511554 Free PMC article. Review.
Cited by
-
Inhibitors of the Interleukin-1 Receptor Accessory Protein Signaling: Another Asset in the Cardio-Immunology Toolbox.Circ Heart Fail. 2024 Dec;17(12):e012244. doi: 10.1161/CIRCHEARTFAILURE.124.012244. Epub 2024 Nov 8. Circ Heart Fail. 2024. PMID: 39513252 No abstract available.
-
Pyroptosis in cardiovascular diseases: roles, mechanisms, and clinical implications.Front Cardiovasc Med. 2025 Aug 4;12:1629016. doi: 10.3389/fcvm.2025.1629016. eCollection 2025. Front Cardiovasc Med. 2025. PMID: 40832143 Free PMC article. Review.
References
-
- Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–48. doi: 10.1093/eurheartj/eht210. - DOI - PubMed
-
- Wojnicz R, Nowalany-Kozielska E, Wojciechowska C, Glanowska G, Wilczewski P, Niklewski T, et al. Randomized, placebo-controlled study for immunosuppressive treatment of inflammatory dilated cardiomyopathy: two-year follow-up results. Circulation. 2001;104:39–45. doi: 10.1161/01.CIR.104.1.39. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

