Immunogenicity and safety of live attenuated and recombinant/inactivated varicella zoster vaccines in people living with HIV: A systematic review
- PMID: 38650460
- PMCID: PMC11042063
- DOI: 10.1080/21645515.2024.2341456
Immunogenicity and safety of live attenuated and recombinant/inactivated varicella zoster vaccines in people living with HIV: A systematic review
Abstract
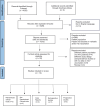
Few papers focus their attention on VZV vaccination effectiveness among people living with HIV (PLWH). Flanking the live attenuated vaccine (VZL) available, a newly recombinant vaccine (RZV) was recently introduced and approved for HZ prevention among adults. PLWH represents a population on which a particular attention should be applied, in order to guarantee the vaccine efficacy and safety. We performed a literature search in USNLM, PubMed, PubMed Central, PMC and Cochrane Library. From all the publications found eligible, data were extracted and processed per population, vaccine type, immunogenicity and ADRs. The review of the 13 included studies shows that both RZV and VZL are immunogenic and have an acceptable safety profile in adults and children living with HIV. However, given the lack of research available about vaccine efficacy in preventing VZV and HZ in PLWH, additional studies need to be performed, in order to achieve a full completeness of data.
Keywords: Vaccine; herpes zoster; immunogenicity; safety; varicella.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
References
-
- Abendroth A, Arvin AM, Moffat JF. Varicella-zoster virus. Springer Science & Business Media; 2010.
-
- Marin M, Guris D, Chaves SS, Schmid S, Seward JF. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practice (ACIP). Vol. 56, MMWR Recomm Rep; 2007. pp. 1–40. - PubMed
-
- World Health Organization . Varicella and herpes zoster vaccines: WHO position Paper. Wkly Epidemiol Rec. 2014;25:265. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical