The impact of the senescent microenvironment on tumorigenesis: Insights for cancer therapy
- PMID: 38650467
- PMCID: PMC11113271
- DOI: 10.1111/acel.14182
The impact of the senescent microenvironment on tumorigenesis: Insights for cancer therapy
Abstract
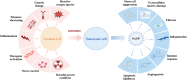
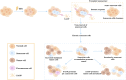
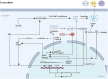
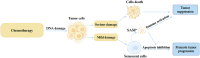
The growing global burden of cancer, especially among people aged 60 years and over, has become a key public health issue. This trend suggests the need for a deeper understanding of the various cancer types in order to develop universally effective treatments. A prospective area of research involves elucidating the interplay between the senescent microenvironment and tumor genesis. Currently, most oncology research focuses on adulthood and tends to ignore the potential role of senescent individuals on tumor progression. Senescent cells produce a senescence-associated secretory phenotype (SASP) that has a dual role in the tumor microenvironment (TME). While SASP components can remodel the TME and thus hinder tumor cell proliferation, they can also promote tumorigenesis and progression via pro-inflammatory and pro-proliferative mechanisms. To address this gap, our review seeks to investigate the influence of senescent microenvironment changes on tumor development and their potential implications for cancer therapies.
Keywords: cancer; senescence‐associated secretory phenotype; senescent; tumor microenvironment.
© 2024 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures
Similar articles
-
Reshaping of the tumor microenvironment by cellular senescence: An opportunity for senotherapies.Dev Cell. 2023 Jun 19;58(12):1007-1021. doi: 10.1016/j.devcel.2023.05.010. Dev Cell. 2023. PMID: 37339603 Review.
-
Targeting senescent cells to reshape the tumor microenvironment and improve anticancer efficacy.Semin Cancer Biol. 2024 Jun;101:58-73. doi: 10.1016/j.semcancer.2024.05.002. Epub 2024 May 27. Semin Cancer Biol. 2024. PMID: 38810814 Review.
-
Cellular senescence and the tumour microenvironment.Mol Oncol. 2022 Sep;16(18):3333-3351. doi: 10.1002/1878-0261.13268. Epub 2022 Jun 26. Mol Oncol. 2022. PMID: 35674109 Free PMC article. Review.
-
The role of cellular senescence and SASP in tumour microenvironment.FEBS J. 2023 Mar;290(5):1348-1361. doi: 10.1111/febs.16381. Epub 2022 Feb 20. FEBS J. 2023. PMID: 35106956 Review.
-
Targeting senescence-associated secretory phenotypes to remodel the tumour microenvironment and modulate tumour outcomes.Clin Transl Med. 2024 Sep;14(9):e1772. doi: 10.1002/ctm2.1772. Clin Transl Med. 2024. PMID: 39270064 Free PMC article. Review.
Cited by
-
ATM and p53 in aging and cancer: a double-edged sword in genomic integrity.Biogerontology. 2025 May 5;26(3):102. doi: 10.1007/s10522-025-10249-4. Biogerontology. 2025. PMID: 40323544 Review.
-
Unraveling senescence in cancer: mechanistic complexities and therapeutic opportunities.Mol Biol Rep. 2025 May 30;52(1):521. doi: 10.1007/s11033-025-10630-z. Mol Biol Rep. 2025. PMID: 40445455 Review.
-
Cellular senescence in cancer: from mechanism paradoxes to precision therapeutics.Mol Cancer. 2025 Aug 8;24(1):213. doi: 10.1186/s12943-025-02419-2. Mol Cancer. 2025. PMID: 40781676 Free PMC article. Review.
-
Mechanical forces in the tumor microenvironment: roles, pathways, and therapeutic approaches.J Transl Med. 2025 Mar 12;23(1):313. doi: 10.1186/s12967-025-06306-8. J Transl Med. 2025. PMID: 40075523 Free PMC article. Review.
References
-
- Acosta, J. C. , Banito, A. , Wuestefeld, T. , Georgilis, A. , Janich, P. , Morton, J. P. , Athineos, D. , Kang, T. W. , Lasitschka, F. , Andrulis, M. , Pascual, G. , Morris, K. J. , Khan, S. , Jin, H. , Dharmalingam, G. , Snijders, A. P. , Carroll, T. , Capper, D. , Pritchard, C. , … Gil, J. (2013). A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nature Cell Biology, 15(8), 978–990. - PMC - PubMed
-
- Adjogatse, D. , Thanopoulou, E. , Okines, A. , Thillai, K. , Tasker, F. , Johnston, S. R. D. , Harper‐Wynne, C. , Torrisi, E. , & Ring, A. (2014). Febrile neutropaenia and chemotherapy discontinuation in women aged 70 years or older receiving adjuvant chemotherapy for early breast cancer. Clinical Oncology (Royal College of Radiologists), 26(11), 692–696. - PubMed
-
- Althubiti, M. , Lezina, L. , Carrera, S. , Jukes‐Jones, R. , Giblett, S. M. , Antonov, A. , Barlev, N. , Saldanha, G. S. , Pritchard, C. A. , Cain, K. , & Macip, S. (2014). Characterization of novel markers of senescence and their prognostic potential in cancer. Cell Death & Disease, 5(11), e1528. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

