Effect of P. corylifolia on the pharmacokinetic profile of tofacitinib and the underlying mechanism
- PMID: 38650629
- PMCID: PMC11033359
- DOI: 10.3389/fphar.2024.1351882
Effect of P. corylifolia on the pharmacokinetic profile of tofacitinib and the underlying mechanism
Abstract
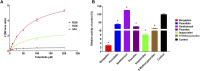
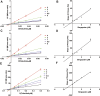
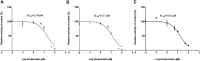
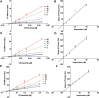
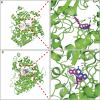
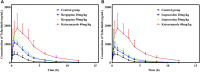
This work aimed to explore the mechanisms underlying the interaction of the active furanocoumarins in P. corylifolia on tofacitinib both in vivo and in vitro. The concentration of tofacitinib and its metabolite M8 was determined using UPLC-MS/MS. The peak area ratio of M8 to tofacitinib was calculated to compare the inhibitory ability of furanocoumarin contained in the traditional Chinese medicine P. corylifolia in rat liver microsomes (RLMs), human liver microsomes (HLMs) and recombinant human CYP3A4 (rCYP3A4). We found that bergapten and isopsoralen exhibited more significant inhibitory activity in RLMs than other furanocoumarins. Bergapten and isopsoralen were selected to investigate tofacitinib drug interactions in vitro and in vivo. Thirty rats were randomly allocated into 5 groups (n = 6): control (0.5% CMC-Na), low-dose bergapten (20 mg/kg), high-dose bergapten (50 mg/kg), low-dose isopsoralen (20 mg/kg) and ketoconazole. 10 mg/kg of tofacitinib was orally intervented to each rat and the concentration level of tofacitinib in the rats were determined by UPLC-MS/MS. More imporrantly, the results showed that bergapten and isopsoralen significantly inhibited the metabolism of tofacitinib metabolism. The AUC(0-t), AUC(0-∞), MRT(0-t), MRT(0-∞) and Cmax of tofacitinib increased in varying degrees compared with the control group (all p < 0.05), but CLz/F decreased in varying degrees (p < 0.05) in the different dose bergapten group and isopsoralen group. Bergapten, isopsoralen and tofacitinib exhibit similar binding capacities with CYP3A4 by AutoDock 4.2 software, confirming that they compete for tofacitinib metabolism. P. corylifolia may considerably impact the metabolism of tofacitinib, which can provide essential information for the accurate therapeutic application of tofacitinib.
Keywords: P. corylifolia; cytochrome P450; drug-drug interaction; molecular docking; pharmacokinetics.
Copyright © 2024 Wang, Zhou, Wang, Song, Wang, Mamun, Geng, Zhou and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Effects of bergapten on the pharmacokinetics of macitentan in rats both in vitro and in vivo.Front Pharmacol. 2023 Jul 10;14:1204649. doi: 10.3389/fphar.2023.1204649. eCollection 2023. Front Pharmacol. 2023. PMID: 37492094 Free PMC article.
-
A UPLC-MS/MS method for in vivo and in vitro pharmacokinetic studies of psoralenoside, isopsoralenoside, psoralen and isopsoralen from Psoralea corylifolia extract.J Ethnopharmacol. 2014;151(1):609-17. doi: 10.1016/j.jep.2013.11.013. Epub 2013 Dec 4. J Ethnopharmacol. 2014. PMID: 24315982
-
The Impact of Baohuoside I on the Metabolism of Tofacitinib in Rats.Drug Des Devel Ther. 2024 Mar 25;18:931-939. doi: 10.2147/DDDT.S436549. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 38560524 Free PMC article.
-
The effect of Resveratrol on the pharmacokinetic profile of tofacitinib and the underlying mechanism.Chem Biol Interact. 2023 Apr 1;374:110398. doi: 10.1016/j.cbi.2023.110398. Epub 2023 Feb 9. Chem Biol Interact. 2023. PMID: 36773832
-
Effects of Avitinib on CYP450 Enzyme Activity in vitro and in vivo in Rats.Drug Des Devel Ther. 2021 Aug 21;15:3661-3673. doi: 10.2147/DDDT.S323186. eCollection 2021. Drug Des Devel Ther. 2021. PMID: 34456561 Free PMC article.
Cited by
-
Physiologically Based Pharmacokinetic Modeling of Tofacitinib: Predicting Drug Exposure and Optimizing Dosage in Special Populations and Drug-Drug Interaction Scenarios.Pharmaceuticals (Basel). 2025 Mar 18;18(3):425. doi: 10.3390/ph18030425. Pharmaceuticals (Basel). 2025. PMID: 40143201 Free PMC article.
References
LinkOut - more resources
Full Text Sources

