Effects of Tcte1 knockout on energy chain transportation and spermatogenesis: implications for male infertility
- PMID: 38650655
- PMCID: PMC11035007
- DOI: 10.1093/hropen/hoae020
Effects of Tcte1 knockout on energy chain transportation and spermatogenesis: implications for male infertility
Abstract
Study question: Is the Tcte1 mutation causative for male infertility?
Summary answer: Our collected data underline the complex and devastating effect of the single-gene mutation on the testicular molecular network, leading to male reproductive failure.
What is known already: Recent data have revealed mutations in genes related to axonemal dynein arms as causative for morphology and motility abnormalities in spermatozoa of infertile males, including dysplasia of fibrous sheath (DFS) and multiple morphological abnormalities in the sperm flagella (MMAF). The nexin-dynein regulatory complex (N-DRC) coordinates the dynein arm activity and is built from the DRC1-DRC7 proteins. DRC5 (TCTE1), one of the N-DRC elements, has already been reported as a candidate for abnormal sperm flagella beating; however, only in a restricted manner with no clear explanation of respective observations.
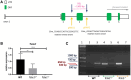
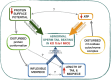
Study design size duration: Using the CRISPR/Cas9 genome editing technique, a mouse Tcte1 gene knockout line was created on the basis of the C57Bl/6J strain. The mouse reproductive potential, semen characteristics, testicular gene expression levels, sperm ATP, and testis apoptosis level measurements were then assessed, followed by visualization of N-DRC proteins in sperm, and protein modeling in silico. Also, a pilot genomic sequencing study of samples from human infertile males (n = 248) was applied for screening of TCTE1 variants.
Participants/materials setting methods: To check the reproductive potential of KO mice, adult animals were crossed for delivery of three litters per caged pair, but for no longer than for 6 months, in various combinations of zygosity. All experiments were performed for wild-type (WT, control group), heterozygous Tcte1+/- and homozygous Tcte1-/- male mice. Gross anatomy was performed on testis and epididymis samples, followed by semen analysis. Sequencing of RNA (RNAseq; Illumina) was done for mice testis tissues. STRING interactions were checked for protein-protein interactions, based on changed expression levels of corresponding genes identified in the mouse testis RNAseq experiments. Immunofluorescence in situ staining was performed to detect the N-DRC complex proteins: Tcte1 (Drc5), Drc7, Fbxl13 (Drc6), and Eps8l1 (Drc3) in mouse spermatozoa. To determine the amount of ATP in spermatozoa, the luminescence level was measured. In addition, immunofluorescence in situ staining was performed to check the level of apoptosis via caspase 3 visualization on mouse testis samples. DNA from whole blood samples of infertile males (n = 137 with non-obstructive azoospermia or cryptozoospermia, n = 111 samples with a spectrum of oligoasthenoteratozoospermia, including n = 47 with asthenozoospermia) was extracted to perform genomic sequencing (WGS, WES, or Sanger). Protein prediction modeling of human-identified variants and the exon 3 structure deleted in the mouse knockout was also performed.
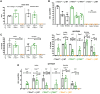
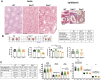
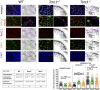
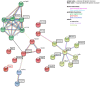
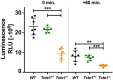
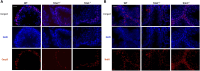
Main results and the role of chance: No progeny at all was found for the homozygous males which were revealed to have oligoasthenoteratozoospermia, while heterozygous animals were fertile but manifested oligozoospermia, suggesting haploinsufficiency. RNA-sequencing of the testicular tissue showed the influence of Tcte1 mutations on the expression pattern of 21 genes responsible for mitochondrial ATP processing or linked with apoptosis or spermatogenesis. In Tcte1-/- males, the protein was revealed in only residual amounts in the sperm head nucleus and was not transported to the sperm flagella, as were other N-DRC components. Decreased ATP levels (2.4-fold lower) were found in the spermatozoa of homozygous mice, together with disturbed tail:midpiece ratios, leading to abnormal sperm tail beating. Casp3-positive signals (indicating apoptosis) were observed in spermatogonia only, at a similar level in all three mouse genotypes. Mutation screening of human infertile males revealed one novel and five ultra-rare heterogeneous variants (predicted as disease-causing) in 6.05% of the patients studied. Protein prediction modeling of identified variants revealed changes in the protein surface charge potential, leading to disruption in helix flexibility or its dynamics, thus suggesting disrupted interactions of TCTE1 with its binding partners located within the axoneme.
Large scale data: All data generated or analyzed during this study are included in this published article and its supplementary information files. RNAseq data are available in the GEO database (https://www.ncbi.nlm.nih.gov/geo/) under the accession number GSE207805. The results described in the publication are based on whole-genome or exome sequencing data which includes sensitive information in the form of patient-specific germline variants. Information regarding such variants must not be shared publicly following European Union legislation, therefore access to raw data that support the findings of this study are available from the corresponding author upon reasonable request.
Limitations reasons for caution: In the study, the in vitro fertilization performance of sperm from homozygous male mice was not checked.
Wider implications of the findings: This study contains novel and comprehensive data concerning the role of TCTE1 in male infertility. The TCTE1 gene is the next one that should be added to the 'male infertility list' because of its crucial role in spermatogenesis and proper sperm functioning.
Study funding/competing interests: This work was supported by National Science Centre in Poland, grants no.: 2015/17/B/NZ2/01157 and 2020/37/B/NZ5/00549 (to M.K.), 2017/26/D/NZ5/00789 (to A.M.), and HD096723, GM127569-03, NIH SAP #4100085736 PA DoH (to A.N.Y.). The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Keywords: Fetub; MMAF; N-DRC; TCTE1; haploinsufficiency; male infertility; mt-Co2; oligoasthenoteratozoospermia; sperm mitochondria; sperm motility.
© The Author(s) 2024. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Figures





Similar articles
-
Whole-exome sequencing identifies mutations in FSIP2 as a recurrent cause of multiple morphological abnormalities of the sperm flagella.Hum Reprod. 2018 Oct 1;33(10):1973-1984. doi: 10.1093/humrep/dey264. Hum Reprod. 2018. PMID: 30137358
-
Whole-exome sequencing of a cohort of infertile men reveals novel causative genes in teratozoospermia that are chiefly related to sperm head defects.Hum Reprod. 2021 Dec 27;37(1):152-177. doi: 10.1093/humrep/deab229. Hum Reprod. 2021. PMID: 34791246
-
Whole-exome sequencing of familial cases of multiple morphological abnormalities of the sperm flagella (MMAF) reveals new DNAH1 mutations.Hum Reprod. 2016 Dec;31(12):2872-2880. doi: 10.1093/humrep/dew262. Epub 2016 Oct 26. Hum Reprod. 2016. PMID: 27798045
-
Molecular genetics of infertility: loss-of-function mutations in humans and corresponding knockout/mutated mice.Hum Reprod Update. 2021 Jan 4;27(1):154-189. doi: 10.1093/humupd/dmaa034. Hum Reprod Update. 2021. PMID: 33118031 Review.
-
[The Roles of N6-Methyladenosine Modification and Its Regulators in Male Reproduction].Sichuan Da Xue Xue Bao Yi Xue Ban. 2024 May 20;55(3):527-534. doi: 10.12182/20240560103. Sichuan Da Xue Xue Bao Yi Xue Ban. 2024. PMID: 38948273 Free PMC article. Review. Chinese.
References
-
- Agarwal A, Baskaran S, Parekh N, Cho CL, Henkel R, Vij S, Arafa M, Panner Selvam MK, Shah R.. Male infertility. Lancet 2021;397:319–333. - PubMed
-
- Alvarez-Paggi D, Hannibal L, Castro MA, Oviedo-Rouco S, Demicheli V, Tórtora V, Tomasina F, Radi R, Murgida DH.. Multifunctional cytochrome C: learning new tricks from an old dog. Chem Rev 2017;117:13382–13460. - PubMed
-
- Anderson MJ, Dixson AF.. Sperm competition: motility and the midpiece in primates. Nature 2002;416:496. - PubMed
-
- Aprea I, Raidt J, Höben IM, Loges NT, Nöthe-Menchen T, Pennekamp P, Olbrich H, Kaiser T, Biebach L, Tüttelmann F. et al. Defects in the cytoplasmic assembly of axonemal dynein arms cause morphological abnormalities and dysmotility in sperm cells leading to male infertility. PLoS Genet 2021;17:e1009306. - PMC - PubMed
-
- Aurrière J, Goudenège D, Baris OR, Boguenet M, May-Panloup P, Lenaers G, Khiati S.. Cancer/testis antigens into mitochondria: a hub between spermatogenesis, tumorigenesis and mitochondrial physiology adaptation. Mitochondrion 2021;56:73–81. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
