γ-Aminobutyric acid transporter and GABAA receptor mechanisms in Slc6a1+/A288V and Slc6a1+/S295L mice associated with developmental and epileptic encephalopathies
- PMID: 38650830
- PMCID: PMC11032196
- DOI: 10.1093/braincomms/fcae110
γ-Aminobutyric acid transporter and GABAA receptor mechanisms in Slc6a1+/A288V and Slc6a1+/S295L mice associated with developmental and epileptic encephalopathies
Abstract
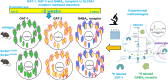
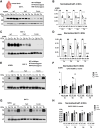
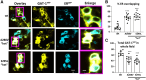
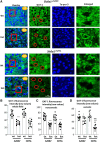
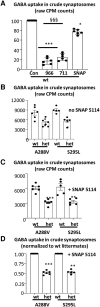
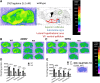
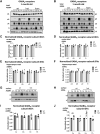
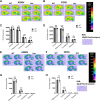
We have previously characterized the molecular mechanisms for variants in γ-aminobutyric acid transporter 1-encoding solute carrier family 6-member 1 (SLC6A1) in vitro and concluded that a partial or complete loss of γ-aminobutyric acid uptake due to impaired protein trafficking is the primary aetiology. Impairment of γ-aminobutyric acid transporter 1 function could cause compensatory changes in the expression of γ-aminobutyric acid receptors, which, in turn, modify disease pathophysiology and phenotype. Here we used different approaches including radioactive 3H γ-aminobutyric acid uptake in cells and synaptosomes, immunohistochemistry and confocal microscopy as well as brain slice surface protein biotinylation to characterize Slc6a1+/A288V and Slc6a1+/S295L mice, representative of a partial or a complete loss of function of SLC6A1 mutations, respectively. We employed the γ-aminobutyric acid transporter 1-specific inhibitor [3H]tiagabine binding and GABAA receptor subunit-specific radioligand binding to profile the γ-aminobutyric acid transporter 1 and GABAA receptor expression in major brain regions such as cortex, cerebellum, hippocampus and thalamus. We also determined the total and surface expression of γ-aminobutyric acid transporter 1, γ-aminobutyric acid transporter 3 and expression of GABAA receptor in the major brain regions in the knockin mice. We found that γ-aminobutyric acid transporter 1 protein was markedly reduced in cortex, hippocampus, thalamus and cerebellum in both mutant mouse lines. Consistent with the findings of reduced γ-aminobutyric acid uptake for both γ-aminobutyric acid transporter 1(A288V) and γ-aminobutyric acid transporter 1(S295L), both the total and the γ-aminobutyric acid transporter 1-mediated 3H γ-aminobutyric acid reuptake was reduced. We found that γ-aminobutyric acid transporter 3 is only abundantly expressed in the thalamus and there was no compensatory increase of γ-aminobutyric acid transporter 3 in either of the mutant mouse lines. γ-Aminobutyric acid transporter 1 was reduced in both somatic regions and nonsomatic regions in both mouse models, in which a ring-like structure was identified only in the Slc6a1+/A288V mouse, suggesting more γ-aminobutyric acid transporter 1 retention inside endoplasmic reticulum in the Slc6a1+/A288V mouse. The [3H]tiagabine binding was similar in both mouse models despite the difference in γ-aminobutyric acid uptake function and γ-aminobutyric acid transporter 1 protein expression for both mutations. There were no differences in GABAA receptor subtype expression, except for a small increase in the expression of α5 subunits of GABAA receptor in the hippocampus of Slc6a1S295L homozygous mice, suggesting a potential interaction between the expression of this GABAA receptor subtype and the mutant γ-aminobutyric acid transporter 1. The study provides the first comprehensive characterization of the SLC6A1 mutations in vivo in two representative mouse models. Because both γ-aminobutyric acid transporter 1 and GABAA receptors are targets for anti-seizure medications, the findings from this study can help guide tailored treatment options based on the expression and function of γ-aminobutyric acid transporter 1 and GABAA receptor in SLC6A1 mutation-mediated neurodevelopmental and epileptic encephalopathies.
Keywords: GABA transporter 1 (GAT-1); GABAA receptors (GABAARs); endoplasmic reticulum retention; epilepsy; neurodevelopmental delay.
Published by Oxford University Press on behalf of the Guarantors of Brain 2024.
Conflict of interest statement
The authors report no competing interests.
Figures

References
-
- Mattison KA, Butler KM, Inglis GAS, et al. SLC6A1 variants identified in epilepsy patients reduce γ-aminobutyric acid transport. Epilepsia. 2018;59(9):e135–e141. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
