High prevalence of response to PPI treatment in children and adolescents with eosinophilic esophagitis in southern Brazil
- PMID: 38650863
- PMCID: PMC11033356
- DOI: 10.3389/falgy.2024.1346843
High prevalence of response to PPI treatment in children and adolescents with eosinophilic esophagitis in southern Brazil
Abstract
Introduction: Eosinophilic esophagitis is a newly recognized entity, in which there is significant evidence available that clearly demonstrates the positive impact of PPIs on reducing esophageal eosinophilia in individuals across different age groups, including children, adolescents, and adults. Multiple mechanisms have been proposed to explain how this treatment effect occurs. In Brazil, there seems to be a lack of studies that have prospectively assessed the clinical and therapeutic response rate in pediatric patients with EoE. The objective of this study was to prospectively evaluate the clinical and therapeutic response of pediatric patients with EoE in a medical center located in southern Brazil, by investigating the effectiveness of PPI treatment.
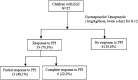
Methods: This study is a clinical, prospective, open trial that took place in a pediatric hospital located in southern Brazil. The focus of the study was on patients diagnosed with Eosinophilic Esophagitis (EoE) who were given treatment using omeprazole/esomeprazole at a dosage of 1 mg.kg per dose, twice daily, for a period of 8-12 weeks. Following the treatment period, the patients underwent another endoscopy. Patients who exhibited 15 or less eosinophils in the biopsy conducted after the treatment were considered as responders.
Results: A total of 27 patients was evaluated (74.1% boys). The average age (± standard deviation) was 8 years (±4). Nineteen patients (70.3%) were considered as responders to PPI treatment: 6 patients-22.2%-exhibited a complete response (defined as having 5 or fewer eosinophil per high power field. Additionally, 13 patients-48.1%-demonstrated a partial response, characterized by eosinophil counts exceeding 5 but less than 15 eos/hpf. When comparing the responder and non-responder groups at presentation, a statistical difference was observed in the prevalence of food refusal as a presenting symptom. Food refusal was found to be more prevalent in the non-responder group (87.5% vs. 26.3%, P = 0.008). No differences were observed in terms of atopy history and endoscopic scores. Upon comparing the histological findings from the post-treatment endoscopy of the two groups, it was observed that PPI responders exhibited a greater tendency to decrease basal cell hyperplasia (P = 0.06) and intercellular edema (P = 0.08).
Conclusion: In this group of pediatric patients with EoE in Southern Brazil most patients showed a high prevalence of histological, endoscopic, and clinical response to PPI treatment. PPIs showed efficacy in Brazilian patients with EoE, most of whom would probably not be able to adequately undergo other treatments.
Clinical trial registration: https://ensaiosclinicos.gov.br/rg/RBR-2ntbth9, identifier (U1111-1301-1842).
Keywords: children; eosinophilic esophagitis; histological remission; proton-pump inhibitor; proton-pump inhibitor responsive esophageal eosinophilia.
© 2024 Nader, Epifanio, Coelho, Steinhaus, Melere, da Silva and Ferreira.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Queiros MC, Bandeira LA, de Souza AEF, de Paiva SF, Alcântara VS, Mouro MF, et al. A prevalência da esofagite eosinofílica no Brasil—uma revisão da literatura. Braz J Dev. (2023) 9(05):16867–76. 10.34117/bjdv9n5-157 - DOI
LinkOut - more resources
Full Text Sources