Abatacept increases T cell exhaustion in early RA individuals who carry HLA risk alleles
- PMID: 38650930
- PMCID: PMC11033422
- DOI: 10.3389/fimmu.2024.1383110
Abatacept increases T cell exhaustion in early RA individuals who carry HLA risk alleles
Abstract
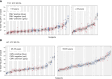
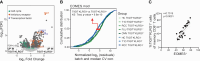
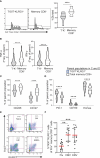
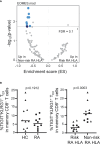
Exhausted CD8 T cells (TEX) are associated with worse outcome in cancer yet better outcome in autoimmunity. Building on our past findings of increased TIGIT+KLRG1+ TEX with teplizumab therapy in type 1 diabetes (T1D), in the absence of treatment we found that the frequency of TIGIT+KLRG1+ TEX is stable within an individual but differs across individuals in both T1D and healthy control (HC) cohorts. This TIGIT+KLRG1+ CD8 TEX population shares an exhaustion-associated EOMES gene signature in HC, T1D, rheumatoid arthritis (RA), and cancer subjects, expresses multiple inhibitory receptors, and is hyporesponsive in vitro, together suggesting co-expression of TIGIT and KLRG1 may broadly define human peripheral exhausted cells. In HC and RA subjects, lower levels of EOMES transcriptional modules and frequency of TIGIT+KLRG1+ TEX were associated with RA HLA risk alleles (DR0401, 0404, 0405, 0408, 1001) even when considering disease status and cytomegalovirus (CMV) seropositivity. Moreover, the frequency of TIGIT+KLRG1+ TEX was significantly increased in RA HLA risk but not non-risk subjects treated with abatacept (CTLA4Ig). The DR4 association and selective modulation with abatacept suggests that therapeutic modulation of TEX may be more effective in DR4 subjects and TEX may be indirectly influenced by cellular interactions that are blocked by abatacept.
Keywords: HLA risk alleles; T cell exhaustion; abatacept; autoimmunity; rheumatoid arthritis.
Copyright © 2024 Long, Muir, Jones, Wall, Ylescupidez, Hocking, Pribitzer, Thorpe, Fuchs, Wiedeman, Tatum, Lambert, Uchtenhagen, Speake, Ng, Heubeck, Torgerson, Savage, Maldonado, Ray, Khaychuk, Liu, Linsley and Buckner.
Conflict of interest statement
SL has past and current research projects sponsored by Janssen and SonomaBio. She is a member of the Type 1 Diabetes TrialNet Study Group. VM is currently employed by The Janssen Pharmaceutical Companies of Johnson & Johnson. HU is currently employed by Anocca AB. VW is currently employed by Notch Therapeutics. JT is currently employed by Bristol Myers Squibb. PL is a consultant for Link Therapeutics. JB is a Scientific Co-Founder and Scientific Advisory Board member of GentiBio, a consultant for Bristol Myers Squibb, Neoleukin Therapeutics and Hotspot Therapeutics, and has past and current research projects sponsored by Amgen, Bristol Myers Squibb, Janssen, Novo Nordisk, and Pfizer. She is a member of the Type 1 Diabetes TrialNet Study Group, a partner of the Allen Institute for Immunology, and a member of the Scientific Advisory Boards for the La Jolla Institute for Allergy and Immunology, Oklahoma Medical Research Foundation, and BMS Immunology. JB also has a patent for tenascin-C autoantigenic epitopes in rheumatoid arthritis. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
References
-
- Beltra JC, Manne S, Abdel-Hakeem MS, Kurachi M, Giles JR, Chen Z, et al. Developmental relationships of four exhausted CD8(+) T cell subsets reveals underlying transcriptional and epigenetic landscape control mechanisms. Immunity. (2020) 52:825–41 e8. doi: 10.1016/j.immuni.2020.04.014 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

