Longitudinal cytokine and multi-modal health data of an extremely severe ME/CFS patient with HSD reveals insights into immunopathology, and disease severity
- PMID: 38650940
- PMCID: PMC11033372
- DOI: 10.3389/fimmu.2024.1369295
Longitudinal cytokine and multi-modal health data of an extremely severe ME/CFS patient with HSD reveals insights into immunopathology, and disease severity
Abstract
Introduction: Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) presents substantial challenges in patient care due to its intricate multisystem nature, comorbidities, and global prevalence. The heterogeneity among patient populations, coupled with the absence of FDA-approved diagnostics and therapeutics, further complicates research into disease etiology and patient managment. Integrating longitudinal multi-omics data with clinical, health,textual, pharmaceutical, and nutraceutical data offers a promising avenue to address these complexities, aiding in the identification of underlying causes and providing insights into effective therapeutics and diagnostic strategies.
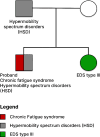
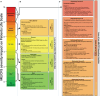
Methods: This study focused on an exceptionally severe ME/CFS patient with hypermobility spectrum disorder (HSD) during a period of marginal symptom improvements. Longitudinal cytokine profiling was conducted alongside the collection of extensive multi-modal health data to explore the dynamic nature of symptoms, severity, triggers, and modifying factors. Additionally, an updated severity assessment platform and two applications, ME-CFSTrackerApp and LexiTime, were introduced to facilitate real-time symptom tracking and enhance patient-physician/researcher communication, and evaluate response to medical intervention.
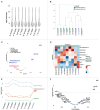
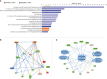
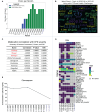
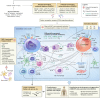
Results: Longitudinal cytokine profiling revealed the significance of Th2-type cytokines and highlighted synergistic activities between mast cells and eosinophils, skewing Th1 toward Th2 immune responses in ME/CFS pathogenesis, particularly in cognitive impairment and sensorial intolerance. This suggests a potentially shared underlying mechanism with major ME/CFS comorbidities such as HSD, Mast cell activation syndrome, postural orthostatic tachycardia syndrome (POTS), and small fiber neuropathy. Additionally, the data identified potential roles of BCL6 and TP53 pathways in ME/CFS etiology and emphasized the importance of investigating adverse reactions to medication and supplements and drug interactions in ME/CFS severity and progression.
Discussion: Our study advocates for the integration of longitudinal multi-omics with multi-modal health data and artificial intelligence (AI) techniques to better understand ME/CFS and its major comorbidities. These findings highlight the significance of dysregulated Th2-type cytokines in patient stratification and precision medicine strategies. Additionally, our results suggest exploring the use of low-dose drugs with partial agonist activity as a potential avenue for ME/CFS treatment. This comprehensive approach emphasizes the importance of adopting a patient-centered care approach to improve ME/CFS healthcare management, disease severity assessment, and personalized medicine. Overall, these findings contribute to our understanding of ME/CFS and offer avenues for future research and clinical practice.
Keywords: EDS/hEDS/HSD; MCAS; MCS; ME/CFS; POTS; Th2-cytokines; complex chronic condition; longitudinal omics.
Copyright © 2024 Jahanbani, Sing, Maynard, Jahanbani, Dafoe, Dafoe, Jones, Wallace, Rastan, Maecker, Röst, Snyder and Davis.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Symptoms of ME/CFS | Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). CDC. Available at: https://www.cdc.gov/me-cfs/symptoms-diagnosis/symptoms.html (Accessed 2 March 2024).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

