Immune modulation in transplant medicine: a comprehensive review of cell therapy applications and future directions
- PMID: 38650942
- PMCID: PMC11033354
- DOI: 10.3389/fimmu.2024.1372862
Immune modulation in transplant medicine: a comprehensive review of cell therapy applications and future directions
Abstract
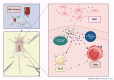
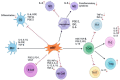
Balancing the immune response after solid organ transplantation (SOT) and vascularized composite allotransplantation (VCA) remains an ongoing clinical challenge. While immunosuppressants can effectively reduce acute rejection rates following transplant surgery, some patients still experience recurrent acute rejection episodes, which in turn may progress to chronic rejection. Furthermore, these immunosuppressive regimens are associated with an increased risk of malignancies and metabolic disorders. Despite significant advancements in the field, these IS related side effects persist as clinical hurdles, emphasizing the need for innovative therapeutic strategies to improve transplant survival and longevity. Cellular therapy, a novel therapeutic approach, has emerged as a potential pathway to promote immune tolerance while minimizing systemic side-effects of standard IS regiments. Various cell types, including chimeric antigen receptor T cells (CAR-T), mesenchymal stromal cells (MSCs), regulatory myeloid cells (RMCs) and regulatory T cells (Tregs), offer unique immunomodulatory properties that may help achieve improved outcomes in transplant patients. This review aims to elucidate the role of cellular therapies, particularly MSCs, T cells, Tregs, RMCs, macrophages, and dendritic cells in SOT and VCA. We explore the immunological features of each cell type, their capacity for immune regulation, and the prospective advantages and obstacles linked to their application in transplant patients. An in-depth outline of the current state of the technology may help SOT and VCA providers refine their perioperative treatment strategies while laying the foundation for further trials that investigate cellular therapeutics in transplantation surgery.
Keywords: SOT; VCA; cellular therapies; solid organ transplantation; vascularized composite allotransplantation.
Copyright © 2024 Knoedler, Dean, Diatta, Thompson, Knoedler, Rhys, Sherwani, Ettl, Mayer, Falkner, Kilian, Panayi, Iske, Safi, Tullius, Haykal, Pomahac and Kauke-Navarro.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer GM declared a shared affiliation, though no other collaboration, with one of the authors JI at the time of the review.
Figures

References
-
- Ruiz R, Kirk AD. Long-term toxicity of immunosuppressive therapy. Transplant Liver. (2015), 1354–63. doi: 10.1016/B978-1-4557-0268-8.00097-X - DOI

