Immunomodulatory effects of a probiotic combination treatment to improve the survival of Pacific oyster (Crassostrea gigas) larvae against infection by Vibrio coralliilyticus
- PMID: 38650950
- PMCID: PMC11033467
- DOI: 10.3389/fimmu.2024.1380089
Immunomodulatory effects of a probiotic combination treatment to improve the survival of Pacific oyster (Crassostrea gigas) larvae against infection by Vibrio coralliilyticus
Erratum in
-
Corrigendum: Immunomodulatory effects of a probiotic combination treatment to improve the survival of Pacific oyster (Crassostrea gigas) larvae against infection by Vibrio coralliilyticus.Front Immunol. 2024 Jul 9;15:1454221. doi: 10.3389/fimmu.2024.1454221. eCollection 2024. Front Immunol. 2024. PMID: 39044813 Free PMC article.
Abstract
Introduction: The culture of Pacific oysters (Crassostrea gigas) is of significant socio-economic importance in the U.S. Pacific Northwest and other temperate regions worldwide, with disease outbreaks acting as significant bottlenecks to the successful production of healthy seed larvae. Therefore, the current study aims to describe the mechanisms of a probiotic combination in improving the survival of C. gigas larvae. Specifically, we investigate changes in C. gigas larval gene expression in response to V. coralliilyticus infection with or without a pre-treatment of a novel probiotic combination.
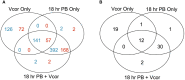
Methods: Treatment groups consisted of replicates of Pacific oyster larvae exposed to a) a combination of four probiotic bacteria at a total concentration of 3.0 x 105 CFU/mL at 18 hours post-fertilization (hpf), b) pathogenic V. coralliilyticus RE22 at a concentration of 6.0 x 103 CFU/mL at 48 hpf, and c) the probiotic combination at 18 hpf and V. coralliilyticus RE22 at 48 hpf. RNA was extracted from washed larvae after 72 hpf, and transcriptome sequencing was used to identify significant differentially expressed genes (DEGs) within each treatment.
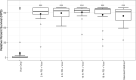
Results: Larvae challenged with V. coralliilyticus showed enhanced expression of genes responsible for inhibiting immune signaling (i.e., TNFAIP3, PSMD10) and inducing apoptosis (i.e., CDIP53). However, when pre-treated with the probiotic combination, these genes were no longer differentially expressed relative to untreated control larvae. Additionally, pre-treatment with the probiotic combination increased expression of immune signaling proteins and immune effectors (i.e., IL-17, MyD88). Apparent immunomodulation in response to probiotic treatment corresponds to an increase in the survival of C. gigas larvae infected with V. coralliilyticus by up to 82%.
Discussion: These results indicate that infection with V. coralliilyticus can suppress the larval immune response while also prompting cell death. Furthermore, the results suggest that the probiotic combination treatment negates the deleterious effects of V. coralliilyticus on larval gene expression while stimulating the expression of genes involved in infection defense mechanisms.
Keywords: Pacific oyster larvae; Vibrio coralliilyticus; aquaculture; immune response; probiotics.
Copyright © 2024 Hesser, Mueller, Langdon and Schubiger.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Botta R, Asche F, Borsum JS, Camp EV. A review of global oyster aquaculture production and consumption. Mar Policy. (2020) 117:103952. doi: 10.1016/j.marpol.2020.103952 - DOI
-
- NOAA Fisheries . Landings (2024). Available online at: https://www.fisheries.noaa.gov/foss/f?p=215:200:25623307448388.
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources

