Transcriptional and Metabolic Response of a Strain of Escherichia coli PTS- to a Perturbation of the Energetic Level by Modification of [ATP]/[ADP] Ratio
- PMID: 38651490
- PMCID: PMC11036233
- DOI: 10.3390/biotech13020010
Transcriptional and Metabolic Response of a Strain of Escherichia coli PTS- to a Perturbation of the Energetic Level by Modification of [ATP]/[ADP] Ratio
Abstract
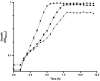
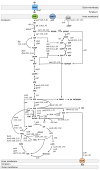
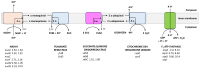
The intracellular [ATP]/[ADP] ratio is crucial for Escherichia coli's cellular functions, impacting transport, phosphorylation, signaling, and stress responses. Overexpression of F1-ATPase genes in E. coli increases glucose consumption, lowers energy levels, and triggers transcriptional responses in central carbon metabolism genes, particularly glycolytic ones, enhancing carbon flux. In this contribution, we report the impact of the perturbation of the energetic level in a PTS- mutant of E. coli by modifying the [ATP]/[ADP] ratio by uncoupling the cytoplasmic activity of the F1 subunit of the ATP synthase. The disruption of [ATP]/[ADP] ratio in the evolved strain of E. coli PB12 (PTS-) was achieved by the expression of the atpAGD operon encoding the soluble portion of ATP synthase F1-ATPase (strain PB12AGD+). The analysis of the physiological and metabolic response of the PTS- strain to the ATP disruption was determined using RT-qPCR of 96 genes involved in glucose and acetate transport, glycolysis and gluconeogenesis, pentose phosphate pathway (PPP), TCA cycle and glyoxylate shunt, several anaplerotic, respiratory chain, and fermentative pathways genes, sigma factors, and global regulators. The apt mutant exhibited reduced growth despite increased glucose transport due to decreased energy levels. It heightened stress response capabilities under glucose-induced energetic starvation, suggesting that the carbon flux from glycolysis is distributed toward the pentose phosphate and the Entner-Duodoroff pathway with the concomitant. Increase acetate transport, production, and utilization in response to the reduction in the [ATP]/[ADP] ratio. Upregulation of several genes encoding the TCA cycle and the glyoxylate shunt as several respiratory genes indicates increased respiratory capabilities, coupled possibly with increased availability of electron donor compounds from the TCA cycle, as this mutant increased respiratory capability by 240% more than in the PB12. The reduction in the intracellular concentration of cAMP in the atp mutant resulted in a reduced number of upregulated genes compared to PB12, suggesting that the mutant remains a robust genetic background despite the severe disruption in its energetic level.
Keywords: Escherichia coli PTS−; F0-F1 ATPase synthase; [ATP]/[ADP] ratio; central carbon metabolism; transcriptional response.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
New insights on transcriptional responses of genes involved in carbon central metabolism, respiration and fermentation to low ATP levels in Escherichia coli.J Basic Microbiol. 2013 Apr;53(4):365-80. doi: 10.1002/jobm.201100525. Epub 2012 Aug 23. J Basic Microbiol. 2013. PMID: 22914992
-
Catabolite regulation analysis of Escherichia coli for acetate overflow mechanism and co-consumption of multiple sugars based on systems biology approach using computer simulation.J Biotechnol. 2013 Oct 20;168(2):155-73. doi: 10.1016/j.jbiotec.2013.06.023. Epub 2013 Jul 10. J Biotechnol. 2013. PMID: 23850830
-
Adaptation for fast growth on glucose by differential expression of central carbon metabolism and gal regulon genes in an Escherichia coli strain lacking the phosphoenolpyruvate:carbohydrate phosphotransferase system.Metab Eng. 2005 Mar;7(2):70-87. doi: 10.1016/j.ymben.2004.10.002. Metab Eng. 2005. PMID: 15781417
-
Metabolic transcription analysis of engineered Escherichia coli strains that overproduce L-phenylalanine.Microb Cell Fact. 2007 Sep 19;6:30. doi: 10.1186/1475-2859-6-30. Microb Cell Fact. 2007. PMID: 17880710 Free PMC article.
-
Carbon catabolite control of the metabolic network in Bacillus subtilis.Biosci Biotechnol Biochem. 2009 Feb;73(2):245-59. doi: 10.1271/bbb.80479. Epub 2009 Feb 7. Biosci Biotechnol Biochem. 2009. PMID: 19202299 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
