Altered serine metabolism promotes drug tolerance in Mycobacterium abscessus via a WhiB7-mediated adaptive stress response
- PMID: 38651855
- PMCID: PMC11620514
- DOI: 10.1128/aac.01456-23
Altered serine metabolism promotes drug tolerance in Mycobacterium abscessus via a WhiB7-mediated adaptive stress response
Abstract
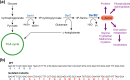
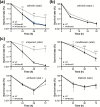
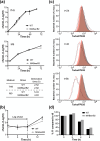
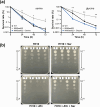
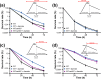
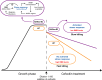
Mycobacterium abscessus is an emerging opportunistic pathogen responsible for chronic lung diseases, especially in patients with cystic fibrosis. Treatment failure of M. abscessus infections is primarily associated with intrinsic or acquired antibiotic resistance. However, there is growing evidence that antibiotic tolerance, i.e., the ability of bacteria to transiently survive exposure to bactericidal antibiotics through physiological adaptations, contributes to the relapse of chronic infections and the emergence of acquired drug resistance. Yet, our understanding of the molecular mechanisms that underlie antibiotic tolerance in M. abscessus remains limited. In the present work, a mutant with increased cross-tolerance to the first- and second-line antibiotics cefoxitin and moxifloxacin, respectively, has been isolated by experimental evolution. This mutant harbors a mutation in serB2, a gene involved in L-serine biosynthesis. Metabolic changes caused by this mutation alter the intracellular redox balance to a more reduced state that induces overexpression of the transcriptional regulator WhiB7 during the stationary phase, promoting tolerance through activation of a WhiB7-dependant adaptive stress response. These findings suggest that alteration of amino acid metabolism and, more generally, conditions that trigger whiB7 overexpression, makes M. abscessus more tolerant to antibiotic treatment.
Keywords: Mycobacterium abscessus; WhiB7; antibiotic tolerance; mycobacteria; serine metabolism; β-lactam.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Balaban NQ, Helaine S, Lewis K, Ackermann M, Aldridge B, Andersson DI, Brynildsen MP, Bumann D, Camilli A, Collins JJ, Dehio C, Fortune S, Ghigo J-M, Hardt W-D, Harms A, Heinemann M, Hung DT, Jenal U, Levin BR, Michiels J, Storz G, Tan M-W, Tenson T, Van Melderen L, Zinkernagel A. 2019. Definitions and guidelines for research on antibiotic persistence. Nat Rev Microbiol 17:441–448. doi:10.1038/s41579-019-0196-3 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

