Recording and classifying MET receptor mutations in cancers
- PMID: 38652103
- PMCID: PMC11042802
- DOI: 10.7554/eLife.92762
Recording and classifying MET receptor mutations in cancers
Abstract
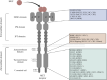
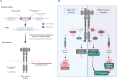
Tyrosine kinase inhibitors (TKI) directed against MET have been recently approved to treat advanced non-small cell lung cancer (NSCLC) harbouring activating MET mutations. This success is the consequence of a long characterization of MET mutations in cancers, which we propose to outline in this review. MET, a receptor tyrosine kinase (RTK), displays in a broad panel of cancers many deregulations liable to promote tumour progression. The first MET mutation was discovered in 1997, in hereditary papillary renal cancer (HPRC), providing the first direct link between MET mutations and cancer development. As in other RTKs, these mutations are located in the kinase domain, leading in most cases to ligand-independent MET activation. In 2014, novel MET mutations were identified in several advanced cancers, including lung cancers. These mutations alter splice sites of exon 14, causing in-frame exon 14 skipping and deletion of a regulatory domain. Because these mutations are not located in the kinase domain, they are original and their mode of action has yet to be fully elucidated. Less than five years after the discovery of such mutations, the efficacy of a MET TKI was evidenced in NSCLC patients displaying MET exon 14 skipping. Yet its use led to a resistance mechanism involving acquisition of novel and already characterized MET mutations. Furthermore, novel somatic MET mutations are constantly being discovered. The challenge is no longer to identify them but to characterize them in order to predict their transforming activity and their sensitivity or resistance to MET TKIs, in order to adapt treatment.
Keywords: cancer; cancer biology; mutation; receptor tyrosine kinase; targeted therapies.
© 2024, Guérin and Tulasne.
Conflict of interest statement
CG, DT No competing interests declared
Figures

References
-
- Abella JV, Peschard P, Naujokas MA, Lin T, Saucier C, Urbé S, Park M. Met/Hepatocyte growth factor receptor ubiquitination suppresses transformation and is required for Hrs phosphorylation. Molecular and Cellular Biology. 2005;25:9632–9645. doi: 10.1128/MCB.25.21.9632-9645.2005. - DOI - PMC - PubMed
-
- Asaoka Y, Tada M, Ikenoue T, Seto M, Imai M, Miyabayashi K, Yamamoto K, Yamamoto S, Kudo Y, Mohri D, Isomura Y, Ijichi H, Tateishi K, Kanai F, Ogawa S, Omata M, Koike K. Gastric cancer cell line hs746t harbors a splice site mutation of c-met causing juxtamembrane domain deletion. Biochemical and Biophysical Research Communications. 2010;394:1042–1046. doi: 10.1016/j.bbrc.2010.03.120. - DOI - PubMed
-
- Awad MM, Bahcall M, Sholl LM, Wilson FH, Paweletz C, Capelletti M, Leonardi GC, Watanabe M, Baba H, Chambers ES, Redig AJ, Nishino M, VanderLaan PA, Costa DB, Imamura Y, Janne PA. Mechanisms of acquired resistance to met tyrosine kinase inhibitors (tkis) in met exon 14 (metex14) mutant non-small cell lung cancer (nsclc) Journal of Clinical Oncology. 2018;36:9069. doi: 10.1200/JCO.2018.36.15_suppl.9069. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

