Non-apoptotic regulated cell death mediates reprogramming of the tumour immune microenvironment by macrophages
- PMID: 38652105
- PMCID: PMC11037416
- DOI: 10.1111/jcmm.18348
Non-apoptotic regulated cell death mediates reprogramming of the tumour immune microenvironment by macrophages
Abstract
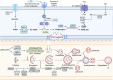
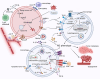
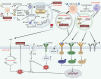
Tumour immune microenvironment (TIME) plays an indispensable role in tumour progression, and tumour-associated macrophages (TAMs) are the most abundant immune cells in TIME. Non-apoptotic regulated cell death (RCD) can avoid the influence of tumour apoptosis resistance on anti-tumour immune response. Specifically, autophagy, ferroptosis, pyroptosis and necroptosis mediate the crosstalk between TAMs and tumour cells in TIME, thus reprogram TIME and affect the progress of tumour. In addition, although some achievements have been made in immune checkpoint inhibitors (ICIs), there is still defect that ICIs are only effective for some people because non-apoptotic RCD can bypass the apoptosis resistance of tumour. As a result, ICIs combined with targeting non-apoptotic RCD may be a promising solution. In this paper, the basic molecular mechanism of non-apoptotic RCD, the way in which non-apoptotic RCD mediates crosstalk between TAMs and tumour cells to reprogram TIME, and the latest research progress in targeting non-apoptotic RCD and ICIs are reviewed.
Keywords: autophagy; ferroptosis; immunotherapy; necroptosis; non‐apoptotic regulated cell death; pyroptosis; tumour immune microenvironment; tumour‐associated macrophages.
© 2024 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Targeting novel regulated cell death: Ferroptosis, pyroptosis and necroptosis in anti-PD-1/PD-L1 cancer immunotherapy.Cell Prolif. 2024 Aug;57(8):e13644. doi: 10.1111/cpr.13644. Epub 2024 Apr 9. Cell Prolif. 2024. PMID: 38594879 Free PMC article. Review.
-
Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor immunotherapy.Signal Transduct Target Ther. 2022 Jun 20;7(1):196. doi: 10.1038/s41392-022-01046-3. Signal Transduct Target Ther. 2022. PMID: 35725836 Free PMC article. Review.
-
A comprehensive investigation of associations between cell death pathways and molecular and clinical features in pan-cancer.Clin Transl Oncol. 2025 Jun;27(6):2731-2749. doi: 10.1007/s12094-024-03769-x. Epub 2024 Nov 2. Clin Transl Oncol. 2025. PMID: 39487950
-
Nanoformula Design for Inducing Non-Apoptotic Cell Death Regulation: A Powerful Booster for Cancer Immunotherapy.Adv Healthc Mater. 2025 Jan;14(2):e2403493. doi: 10.1002/adhm.202403493. Epub 2024 Dec 4. Adv Healthc Mater. 2025. PMID: 39632361 Review.
-
Ferroptosis, necroptosis, and pyroptosis in anticancer immunity.J Hematol Oncol. 2020 Aug 10;13(1):110. doi: 10.1186/s13045-020-00946-7. J Hematol Oncol. 2020. PMID: 32778143 Free PMC article. Review.
Cited by
-
Ultrasound-Triggered NPC1L1-Targeting Nanobubbles for Remodeling the Tumor Microenvironment in Pancreatic Cancer Chemoimmunotherapy.ACS Appl Mater Interfaces. 2025 Jun 18;17(24):34965-34981. doi: 10.1021/acsami.5c01194. Epub 2025 Jun 6. ACS Appl Mater Interfaces. 2025. PMID: 40478203 Free PMC article.
-
Dual roles of inflammatory programmed cell death in cancer: insights into pyroptosis and necroptosis.Front Pharmacol. 2024 Aug 27;15:1446486. doi: 10.3389/fphar.2024.1446486. eCollection 2024. Front Pharmacol. 2024. PMID: 39257400 Free PMC article. Review.
-
Ferroptosis in Pulmonary Disease and Lung Cancer: Molecular Mechanisms, Crosstalk Regulation, and Therapeutic Strategies.MedComm (2020). 2025 Feb 23;6(3):e70116. doi: 10.1002/mco2.70116. eCollection 2025 Mar. MedComm (2020). 2025. PMID: 39991627 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
- 82172989/National Natural Science Foundation of China
- 82273068/National Natural Science Foundation of China
- 82260524/National Natural Science Foundation of China
- 20204343/Project Fund of Jiangxi Provincial Health Com-mission
- 202130873/Project Fund of Jiangxi Provincial Health Com-mission
- 202210044/Project Fund of Jiangxi Provincial Health Com-mission
- 20212BCJ23023/Jiangxi Training Pro-gram for academic and technical leaders of major disciplines-Young talents program
- 20232BAB206101/Jiangxi Provincial Natural Science Foundation
- 2023ZD003/Key project of Science and Technology Innovation of Health Commission
- 20212BBG73021/Key Research and Development projects in Jiangxi
- 2021YNFY12010/The Second Affiliated Hospital of Nanchang University, National Natural Science Foundation incubation project
- GJJ210177/Jiangxi Province Department of Education Science and technology research project, China
LinkOut - more resources
Full Text Sources
Medical
Research Materials

