BRG1 programs PRC2-complex repression and controls oligodendrocyte differentiation and remyelination
- PMID: 38652118
- PMCID: PMC11040499
- DOI: 10.1083/jcb.202310143
BRG1 programs PRC2-complex repression and controls oligodendrocyte differentiation and remyelination
Abstract
Chromatin-remodeling protein BRG1/SMARCA4 is pivotal for establishing oligodendrocyte (OL) lineage identity. However, its functions for oligodendrocyte-precursor cell (OPC) differentiation within the postnatal brain and during remyelination remain elusive. Here, we demonstrate that Brg1 loss profoundly impairs OPC differentiation in the brain with a comparatively lesser effect in the spinal cord. Moreover, BRG1 is critical for OPC remyelination after injury. Integrative transcriptomic/genomic profiling reveals that BRG1 exhibits a dual role by promoting OPC differentiation networks while repressing OL-inhibitory cues and proneuronal programs. Furthermore, we find that BRG1 interacts with EED/PRC2 polycomb-repressive-complexes to enhance H3K27me3-mediated repression at gene loci associated with OL-differentiation inhibition and neurogenesis. Notably, BRG1 depletion decreases H3K27me3 deposition, leading to the upregulation of BMP/WNT signaling and proneurogenic genes, which suppresses OL programs. Thus, our findings reveal a hitherto unexplored spatiotemporal-specific role of BRG1 for OPC differentiation in the developing CNS and underscore a new insight into BRG1/PRC2-mediated epigenetic regulation that promotes and safeguards OL lineage commitment and differentiation.
© 2024 Wang et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures



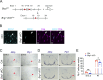


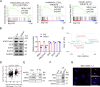
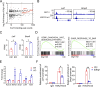

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

