Comparative investigation of neoadjuvant immunotherapy versus adjuvant immunotherapy in perioperative patients with cancer: a global-scale, cross-sectional, and large-sample informatics study
- PMID: 38652128
- PMCID: PMC11325894
- DOI: 10.1097/JS9.0000000000001479
Comparative investigation of neoadjuvant immunotherapy versus adjuvant immunotherapy in perioperative patients with cancer: a global-scale, cross-sectional, and large-sample informatics study
Abstract
Background: Neoadjuvant and adjuvant immunotherapies for cancer have evolved through a series of remarkable and critical research advances; however, addressing their similarities and differences is imperative in clinical practice. Therefore, this study aimed to examine their similarities and differences from the perspective of informatics analysis.
Methods: This cross-sectional study retrospectively analyzed extensive relevant studies published between 2014 and 2023 using stringent search criteria, excluding nonpeer-reviewed and non-English documents. The main outcome variables are publication volume, citation volume, connection strength, occurrence frequency, relevance percentage, and development percentage. Furthermore, an integrated comparative analysis was conducted using unsupervised hierarchical clustering, spatiotemporal analysis, regression statistics, and Walktrap algorithm analysis.
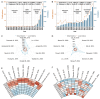
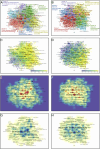
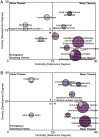
Results: This analysis included 1373 relevant studies. Advancements in neoadjuvant and adjuvant immunotherapies have been promising over the last decade, with an annual growth rate of 25.18 vs. 6.52% and global collaboration (International Co-authorships) of 19.93 vs. 19.84%. Respectively, five dominant research clusters were identified through unsupervised hierarchical clustering based on machine learning, among which Cluster 4 (Balance of neoadjuvant immunotherapy efficacy and safety) and Cluster 2 (Adjuvant immunotherapy clinical trials) [Average Publication Year (APY): 2021.70±0.70 vs. 2017.54±4.59] are emerging research populations. Burst and regression curve analyses uncovered domain pivotal research signatures, including microsatellite instability (R 2 =0.7500, P =0.0025) and biomarkers (R 2 =0.6505, P =0.0086) in neoadjuvant scenarios, and the tumor microenvironment (R 2 =0.5571, P =0.0209) in adjuvant scenarios. The Walktrap algorithm further revealed that 'neoadjuvant immunotherapy, nonsmall cell lung cancer (NSCLC), immune checkpoint inhibitors, melanoma' and 'adjuvant immunotherapy, melanoma, hepatocellular carcinoma, dendritic cells' (Relevance Percentage: 100 vs. 100%, Development Percentage: 37.5 vs. 17.1%) are extremely relevant to this field but remain underdeveloped, highlighting the need for further investigation.
Conclusion: This study identified pivotal research signatures and provided substantial predictions for neoadjuvant and adjuvant cancer immunotherapies. In addition, comprehensive quantitative comparisons revealed a notable shift in focus within this field, with neoadjuvant immunotherapy taking precedence over adjuvant immunotherapy after 2020; such a qualitative finding facilitate proper decision-making for subsequent research and mitigate the wastage of healthcare resources.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
All authors declare that there is no potential commercial or financial interest in this study.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

