Bortezomib suppresses acute myelogenous leukaemia stem-like KG-1a cells via NF-κB inhibition and the induction of oxidative stress
- PMID: 38652192
- PMCID: PMC11037403
- DOI: 10.1111/jcmm.18333
Bortezomib suppresses acute myelogenous leukaemia stem-like KG-1a cells via NF-κB inhibition and the induction of oxidative stress
Abstract
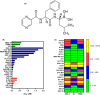
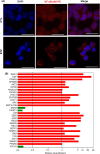
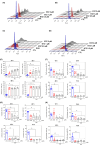
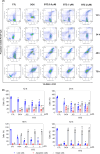
Acute myelogenous leukaemia (AML) originates and is maintained by leukaemic stem cells (LSCs) that are inherently resistant to antiproliferative therapies, indicating that a critical strategy for overcoming chemoresistance in AML therapy is to eradicate LSCs. In this work, we investigated the anti-AML activity of bortezomib (BTZ), emphasizing its anti-LSC potential, using KG-1a cells, an AML cell line with stem-like properties. BTZ presented potent cytotoxicity to both solid and haematological malignancy cells and reduced the stem-like features of KG-1a cells, as observed by the reduction in CD34- and CD123-positive cells. A reduction in NF-κB p65 nuclear staining was observed in BTZ-treated KG-1a cells, in addition to upregulation of the NF-κB inhibitor gene NFΚBIB. BTZ-induced DNA fragmentation, nuclear condensation, cell shrinkage and loss of transmembrane mitochondrial potential along with an increase in active caspase-3 and cleaved PARP-(Asp 214) level in KG-1a cells. Furthermore, BTZ-induced cell death was partially prevented by pretreatment with the pancaspase inhibitor Z-VAD-(OMe)-FMK, indicating that BTZ induces caspase-mediated apoptosis. BTZ also increased mitochondrial superoxide levels in KG-1a cells, and BTZ-induced apoptosis was partially prevented by pretreatment with the antioxidant N-acetylcysteine, indicating that BTZ induces oxidative stress-mediated apoptosis in KG-1a cells. At a dosage of 0.1 mg/kg every other day for 2 weeks, BTZ significantly reduced the percentage of hCD45-positive cells in the bone marrow and peripheral blood of NSG mice engrafted with KG-1a cells with tolerable toxicity. Taken together, these data indicate that the anti-LSC potential of BTZ appears to be an important strategy for AML treatment.
Keywords: AML; NF‐κB; bortezomib; leukaemic stem cells; oxidative stress.
© 2024 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflicts of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

