Exploring the impact of PDGFD in osteosarcoma metastasis through single-cell sequencing analysis
- PMID: 38652223
- PMCID: PMC11467127
- DOI: 10.1007/s13402-024-00949-3
Exploring the impact of PDGFD in osteosarcoma metastasis through single-cell sequencing analysis
Abstract
Purpose: The overall survival rate for metastatic osteosarcoma hovers around 20%. Responses to second-line chemotherapy, targeted therapies, and immunotherapies have demonstrated limited efficacy in metastatic osteosarcoma. Our objective is to validate differentially expressed genes and signaling pathways between non-metastatic and metastatic osteosarcoma, employing single-cell RNA sequencing (scRNA-seq) and additional functional investigations. We aim to enhance comprehension of metastatic mechanisms and potentially unveil a therapeutic target.
Methods: scRNA-seq was performed on two primary osteosarcoma lesions (1 non-metastatic and 1 metastatic). Seurat package facilitated dimensionality reduction and cluster identification. Copy number variation (CNV) was predicted using InferCNV. CellChat characterized ligand-receptor-based intercellular communication networks. Differentially expressed genes underwent GO function enrichment analysis and GSEA. Validation was achieved through the GSE152048 dataset, which identified PDGFD-PDGFRB as a common ligand-receptor pair with significant contribution. Immunohistochemistry assessed PDGFD and PDGFRB expression, while multicolor immunofluorescence and flow cytometry provided insight into spatial relationships and the tumor immune microenvironment. Kaplan-Meier survival analysis compared metastasis-free survival and overall survival between high and low levels of PDGFD and PDGFRB. Manipulation of PDGFD expression in primary osteosarcoma cells examined invasion abilities and related markers.
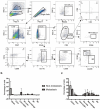
Results: Ten clusters encompassing osteoblasts, osteoclasts, osteocytes, fibroblasts, pericytes, endothelial cells, myeloid cells, T cells, B cells, and proliferating cells were identified. Osteoblasts, osteoclasts, and osteocytes exhibited heightened CNV levels. Ligand-receptor-based communication networks exposed significant fibroblast crosstalk with other cell types, and the PDGF signaling pathway was activated in non-metastatic osteosarcoma primary lesion. These results were corroborated by the GSE152048 dataset, confirming the prominence of PDGFD-PDGFRB as a common ligand-receptor pair. Immunohistochemistry demonstrated considerably greater PDGFD expression in non-metastatic osteosarcoma tissues and organoids, correlating with extended metastasis-free and overall survival. PDGFRB expression showed no significant variation between non-metastatic and metastatic osteosarcoma, nor strong correlations with survival times. Multicolor immunofluorescence suggested co-localization of PDGFD with PDGFRB. Flow cytometry unveiled a highly immunosuppressive microenvironment in metastatic osteosarcoma. Manipulating PDGFD expression demonstrated altered invasive abilities and marker expressions in primary osteosarcoma cells from both non-metastatic and metastatic lesions.
Conclusions: scRNA-seq illuminated the activation of the PDGF signaling pathway in primary lesion of non-metastatic osteosarcoma. PDGFD displayed an inhibitory effect on osteosarcoma metastasis, likely through the suppression of the EMT signaling pathway.
Keywords: Metastasis; Organoid; Osteosarcoma; PDGFD; scRNA-seq.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- H. Koksal, E. Muller, E.M. Inderberg et al., Treating osteosarcoma with CAR T cells. Scand. J. Immunol. 89, e12741 (2019) - PubMed
-
- G. Bacci, A. Briccoli, M. Rocca et al., Neoadjuvant chemotherapy for osteosarcoma of the extremities with metastases at presentation: recent experience at the Rizzoli Institute in 57 patients treated with cisplatin, doxorubicin, and a high dose of methotrexate and ifosfamide. Ann. Oncol. 14, 1126–1134 (2003) - PubMed
-
- A.M. Goorin, M.B. Harris, M. Bernstein et al., Phase II/III trial of etoposide and high-dose ifosfamide in newly diagnosed metastatic osteosarcoma: a pediatric oncology group trial. J. Clin. Oncol. 20, 426–433 (2002) - PubMed
MeSH terms
Substances
Grants and funding
- 2022M722115/China Postdoctoral Science Foundation
- No.19ZR1439100/Science and Technology Commission of Shanghai Municipality
- 21ZR1449000/Natural Science Foundation Project of Shanghai Science and Technology Innovation Action Plan
- 585350/St. Baldrick's Foundation
- 82173358/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

