Anti-spike antibody level is associated with the risk of clinical progression among subjects hospitalized with COVID-19 pneumonia: results from a retrospective cohort study
- PMID: 38652224
- PMCID: PMC11289057
- DOI: 10.1007/s15010-024-02250-9
Anti-spike antibody level is associated with the risk of clinical progression among subjects hospitalized with COVID-19 pneumonia: results from a retrospective cohort study
Abstract
Purpose: Antibodies against SARS-CoV-2 spike (anti-S) may confer protection against symptomatic COVID-19. Whether their level predicts progression among those with COVID-19 pneumonia remains unclear.
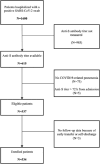
Methods: We conducted a retrospective cohort study to assess predictors of anti-S levels and whether anti-S titer is associated with death or mechanical ventilation (MV). Adults hospitalized for COVID-19 pneumonia between July 2021 and July 2022 were enrolled if anti-S had been measured within 72 h of admission. Predictors of anti-S level were explored using multivariable quantile regression. The association between anti-S levels and 30-day death/MV was investigated via multivariable logistic regression. Analyses were stratified by vaccine status.
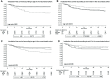
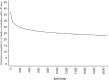
Results: The median anti-S level was 1370 BAU/ml in 328 vaccinated and 15.5 BAU/ml in 206 unvaccinated individuals. Among the vaccinated, shorter symptom duration (p = 0.001), hematological malignancies (p = 0.002), and immunosuppressive therapy (p = 0.004) were associated with lower anti-S levels. In the unvaccinated group, symptom duration was the only predictor of anti-S levels (p < 0.001). After 30 days, 134 patients experienced death or MV. Among vaccinated individuals, higher anti-S levels correlated significantly with lower death/MV risk (per log2 increase, OR 0.88, 95%CI 0.81-0.97), irrespective of age and solid malignancies. Among unvaccinated, a marginally protective effect was observed (OR 0.86, 95%CI 0.73-1.01), independent of age, immunosuppressive therapy, and diabetes. Adjustment for monoclonal antibody treatment strengthened the association (OR 0.81, 95%CI 0.68-0.96).
Conclusion: This study suggests that levels of anti-S antibodies can predict critical or fatal outcomes in COVID-19 pneumonia patients, regardless of vaccination. Whether anti-S Ab could guide risk assessment and vaccination boosting merits further evaluation.
Keywords: Anti-Spike antibody level; COVID-19 pneumonia; Clinical progression; SARS-CoV-2; Vaccination.
© 2024. The Author(s).
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures
Similar articles
-
Antibody levels versus vaccination status in the outcome of older adults with COVID-19.JCI Insight. 2024 Oct 22;9(20):e183913. doi: 10.1172/jci.insight.183913. JCI Insight. 2024. PMID: 39435658 Free PMC article.
-
Seroresponse to SARS-CoV-2 Vaccines among Maintenance Dialysis Patients over 6 Months.Clin J Am Soc Nephrol. 2022 Mar;17(3):403-413. doi: 10.2215/CJN.12250921. Epub 2022 Feb 10. Clin J Am Soc Nephrol. 2022. PMID: 35144972 Free PMC article.
-
SARS-CoV-2 antibody vaccine response in Inflammatory Bowel Disease patients with positive anti-nucleocapsid serology or history of COVID-19 infection.Acta Gastroenterol Belg. 2024 Apr-Jun;87(2):263-273. doi: 10.51821/87.2.12805. Acta Gastroenterol Belg. 2024. PMID: 39210758
-
Response and Duration of Serum Anti-SARS-CoV-2 Antibodies After Inactivated Vaccination Within 160 Days.Front Immunol. 2021 Dec 23;12:786554. doi: 10.3389/fimmu.2021.786554. eCollection 2021. Front Immunol. 2021. PMID: 35003104 Free PMC article.
-
Convalescent Plasma Antibody Levels and the Risk of Death from Covid-19.N Engl J Med. 2021 Mar 18;384(11):1015-1027. doi: 10.1056/NEJMoa2031893. Epub 2021 Jan 13. N Engl J Med. 2021. PMID: 33523609 Free PMC article.
Cited by
-
Clinical outcomes in immunocompromised adults with COVID-19, based on anti-spike IgG serostatus and monoclonal antibody therapy: a retrospective cohort study in the Omicron period.Ther Adv Infect Dis. 2025 Feb 17;12:20499361251320711. doi: 10.1177/20499361251320711. eCollection 2025 Jan-Dec. Ther Adv Infect Dis. 2025. PMID: 39968165 Free PMC article.
-
SARS-CoV-2 vaccination influence in the development of long-COVID clinical phenotypes.Epidemiol Infect. 2025 Feb 4;153:e40. doi: 10.1017/S0950268825000093. Epidemiol Infect. 2025. PMID: 39901510 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

