NR2E3 loss disrupts photoreceptor cell maturation and fate in human organoid models of retinal development
- PMID: 38652563
- PMCID: PMC11142732
- DOI: 10.1172/JCI173892
NR2E3 loss disrupts photoreceptor cell maturation and fate in human organoid models of retinal development
Abstract
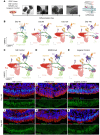
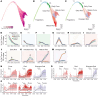
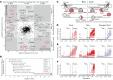
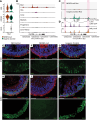
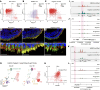
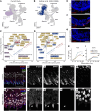
While dysfunction and death of light-detecting photoreceptor cells underlie most inherited retinal dystrophies, knowledge of the species-specific details of human rod and cone photoreceptor cell development remains limited. Here, we generated retinal organoids carrying retinal disease-causing variants in NR2E3, as well as isogenic and unrelated controls. Organoids were sampled using single-cell RNA sequencing (scRNA-Seq) across the developmental window encompassing photoreceptor specification, emergence, and maturation. Using scRNA-Seq data, we reconstruct the rod photoreceptor developmental lineage and identify a branch point unique to the disease state. We show that the rod-specific transcription factor NR2E3 is required for the proper expression of genes involved in phototransduction, including rhodopsin, which is absent in divergent rods. NR2E3-null rods additionally misexpress several cone-specific phototransduction genes. Using joint multimodal single-cell sequencing, we further identify putative regulatory sites where rod-specific factors act to steer photoreceptor cell development. Finally, we show that rod-committed photoreceptor cells form and persist throughout life in a patient with NR2E3-associated disease. Importantly, these findings are strikingly different from those observed in Nr2e3 rodent models. Together, these data provide a road map of human photoreceptor development and leverage patient induced pluripotent stem cells to define the specific roles of rod transcription factors in photoreceptor cell emergence and maturation in health and disease.
Keywords: Development; Genetic diseases; Monogenic diseases; Ophthalmology; iPS cells.
Figures